当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Nonheme FeII/2-Oxoglutarate-Dependent Oxygenase Catalyzes a Double Bond Migration within a Dimethylallyl Moiety Accompanied by Hydroxylation
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-09-18 00:00:00 , DOI: 10.1021/acschembio.8b00588 Huomiao Ran 1 , Viola Wohlgemuth 1 , Xiulan Xie 2 , Shu-Ming Li 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-09-18 00:00:00 , DOI: 10.1021/acschembio.8b00588 Huomiao Ran 1 , Viola Wohlgemuth 1 , Xiulan Xie 2 , Shu-Ming Li 1
Affiliation
|
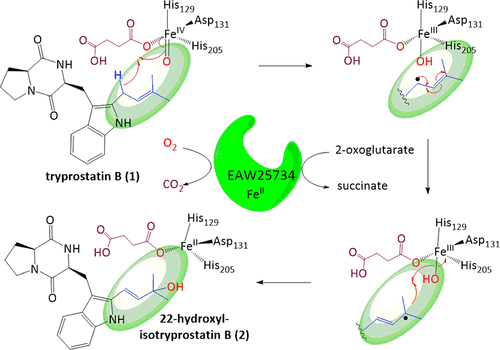
Prenylation of cyclodipeptides contributes largely to the structure diversification and biological activity. The prenylated products can be further metabolized by modifications like hydroxylation with cytochrome P450 enzymes or nonheme FeII/2-oxoglutarate-dependent oxygenases. Herein, we cloned and overexpressed NFIA_045530 from Neosartorya fischeri, which shares high sequence similarity with the nonheme FeII/2-oxoglutarate-dependent oxygenase FtmOx1Af from Aspergillus fumigatus on the amino acid level. FtmOx1Af is a member of the biosynthetic enzymes for fumitremorgin-type mycotoxins and catalyzes the conversion of fumitremorgin B to verruculogen by insertion of an oxygen molecule into the two prenyl moieties. The recombinant protein EAW25734 encoded by NFIA_045530 was purified to apparent homogeneity and then was used for incubation with intermediates of the fumitremorgin biosynthetic pathway. LC-MS analysis revealed no consumption of fumitremorgin B but good conversion with its biosynthetic precursor tryprostatin B in the presence of FeII and 2-oxoglutarate. Structure elucidation confirmed 22-hydroxylisotryprostatin B and 14α, 22-dihydroxylisotryprostatin B as the major enzyme products. Further detailed biochemical characterization led to the identification of a novel enzyme, which catalyzes a double bond migration within the dimethylallyl moiety of tryprostatin B with concomitant hydroxylation. Incubation with 18O2-enriched atmosphere confirmed O2 as the major origin of the hydroxyl groups. Solvent exchange was also observed for that at C22. LC-MS analysis confirmed the presence of 22-hydroxylisotryprostatin B in a Neosartorya fischeri extract, highlighting the role of this enzyme in the metabolism of intermediates of the fumitremorgin/verruculogen pathway. A plausible reaction mechanism implementing a radical rearrangement prior to accepting a hydroxyl radical from FeIII is discussed.
中文翻译:

非血红素Fe II / 2-Oxoglutarate依赖加氧酶催化二甲基烯丙基部分伴随羟基化的双键迁移。
环二肽的烯丙基化主要有助于结构多样化和生物活性。可以通过修饰,例如用细胞色素P450酶或非血红素Fe II / 2-氧代戊二酸酯依赖性加氧酶进行羟基化,使异戊二烯基化产物进一步代谢。在这里,我们克隆和过度表达从费氏新孢子虫的NFIA_045530,其与来自烟曲霉的非血红素Fe II / 2-氧戊二酸依赖性加氧酶FtmOx1 Af在氨基酸水平上具有高度的序列相似性。FtmOx1房颤是Fumitremorgin型霉菌毒素生物合成酶的成员,并且通过将氧分子插入两个异戊二烯基部分来催化Fumitremorgin B转化为疣蛋白原。纯化由NFIA_045530编码的重组蛋白EAW25734,使其具有明显的同质性,然后用于与泛黄褐藻生物合成途径的中间体一起孵育。LC-MS分析表明,在存在Fe II的情况下,未消耗fumitremorgin B,但其生物合成前体Tryprostatin B具有良好的转化率和2-氧戊二酸。结构鉴定证实了22-羟基赖脯氨酸前列腺素B和14α,22-二羟基赖脯氨酸前列腺素B是主要的酶产物。进一步详细的生化表征导致鉴定了一种新型酶,该酶催化胰蛋白酶素B的二甲基烯丙基部分内的双键迁移并伴有羟基化作用。在富含18 O 2的气氛中温育证实了O 2是羟基的主要来源。在C22处也观察到溶剂交换。LC-MS分析确认了费氏新肉球菌中存在22-羟基赖脯前列腺素B提取物,突显了这种酶在褐藻/ verruculogen途径的中间体代谢中的作用。讨论了在接受来自Fe III的羟基自由基之前实施自由基重排的可能的反应机理。
更新日期:2018-09-18
中文翻译:

非血红素Fe II / 2-Oxoglutarate依赖加氧酶催化二甲基烯丙基部分伴随羟基化的双键迁移。
环二肽的烯丙基化主要有助于结构多样化和生物活性。可以通过修饰,例如用细胞色素P450酶或非血红素Fe II / 2-氧代戊二酸酯依赖性加氧酶进行羟基化,使异戊二烯基化产物进一步代谢。在这里,我们克隆和过度表达从费氏新孢子虫的NFIA_045530,其与来自烟曲霉的非血红素Fe II / 2-氧戊二酸依赖性加氧酶FtmOx1 Af在氨基酸水平上具有高度的序列相似性。FtmOx1房颤是Fumitremorgin型霉菌毒素生物合成酶的成员,并且通过将氧分子插入两个异戊二烯基部分来催化Fumitremorgin B转化为疣蛋白原。纯化由NFIA_045530编码的重组蛋白EAW25734,使其具有明显的同质性,然后用于与泛黄褐藻生物合成途径的中间体一起孵育。LC-MS分析表明,在存在Fe II的情况下,未消耗fumitremorgin B,但其生物合成前体Tryprostatin B具有良好的转化率和2-氧戊二酸。结构鉴定证实了22-羟基赖脯氨酸前列腺素B和14α,22-二羟基赖脯氨酸前列腺素B是主要的酶产物。进一步详细的生化表征导致鉴定了一种新型酶,该酶催化胰蛋白酶素B的二甲基烯丙基部分内的双键迁移并伴有羟基化作用。在富含18 O 2的气氛中温育证实了O 2是羟基的主要来源。在C22处也观察到溶剂交换。LC-MS分析确认了费氏新肉球菌中存在22-羟基赖脯前列腺素B提取物,突显了这种酶在褐藻/ verruculogen途径的中间体代谢中的作用。讨论了在接受来自Fe III的羟基自由基之前实施自由基重排的可能的反应机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号