当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insight into selective mechanism of class of I‐BRD9 inhibitors toward BRD9 based on molecular dynamics simulations
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2018-10-08 , DOI: 10.1111/cbdd.13398 Jing Su 1 , Xinguo Liu 1 , Shaolong Zhang 1 , Fangfang Yan 1 , Qinggang Zhang 1 , Jianzhong Chen 2
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2018-10-08 , DOI: 10.1111/cbdd.13398 Jing Su 1 , Xinguo Liu 1 , Shaolong Zhang 1 , Fangfang Yan 1 , Qinggang Zhang 1 , Jianzhong Chen 2
Affiliation
|
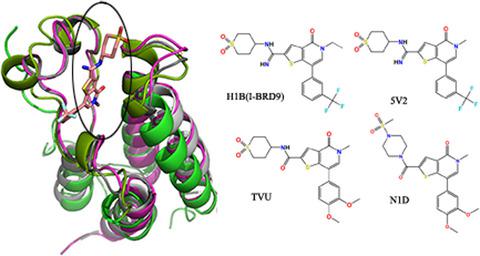
Recently, bromodomain‐containing protein 9 (BRD9), 7 (BRD7), and 4 (BRD4) have been potential targets of anticancer drug design. Molecular dynamic simulations followed by molecular mechanics Poisson–Boltzmann surface area calculation were performed to study the selective mechanism of I‐BRD9 inhibitor H1B and its derivatives N1D, TVU, and 5V2 toward BRD9 and BRD4. The rank of our calculated binding free energies agrees with that of the experimental data. The results show that binding free energy of H1B to BRD7 is slightly lower than that of H1B to BRD9, and all four inhibitors bind more tightly to BRD9 than to BRD4. Decomposition of binding free energies into individual residues implies that Ile164 and Asn211 in BRD7 and Ile53 and Asn100 in BRD9 play a significant role in the selectivity of H1B toward BRD7 and BRD9. Besides, several key residues Phe44, Ile53, Asn100, Thr104 in BRD9 and Pro82, Lys91, Asn140, Asp144 in BRD4 that are located in the ZA‐loop and BC‐loop provide significant contributions to binding selectivity of inhibitors to BRD9 and BRD4. This study is expected to provide important theoretical guidance for rational designs of highly selective inhibitors targeting BRD9 and BRD4.
中文翻译:

基于分子动力学模拟洞察I-BRD9抑制剂类对BRD9的选择性机制
最近,含溴结构域的蛋白9(BRD9),7(BRD7)和4(BRD4)已成为抗癌药物设计的潜在目标。进行分子动力学模拟,然后进行分子力学泊松-玻尔兹曼表面积计算,以研究I-BRD9抑制剂H1B及其衍生物N1D,TVU和5V2对BRD9和BRD4的选择机理。我们计算的结合自由能的等级与实验数据的等级一致。结果表明,H1B与BRD7的结合自由能略低于H1B与BRD9的结合自由能,并且所有四种抑制剂与BRD9的结合比与BRD4的结合更紧密。结合自由能分解成单个残基意味着BRD7中的Ile164和Asn211以及BRD9中的Ile53和Asn100在H1B对BRD7和BRD9的选择性中起重要作用。除了,BRD9中的几个关键残基Phe44,Ile53,Asn100,Thr104和BRD4中的Pro82,Lys91,Asn140,Asp144位于ZA-loop和BC-loop中,这对抑制剂对BRD9和BRD4的结合选择性起了重要作用。预期该研究将为针对BRD9和BRD4的高选择性抑制剂的合理设计提供重要的理论指导。
更新日期:2018-10-08
中文翻译:

基于分子动力学模拟洞察I-BRD9抑制剂类对BRD9的选择性机制
最近,含溴结构域的蛋白9(BRD9),7(BRD7)和4(BRD4)已成为抗癌药物设计的潜在目标。进行分子动力学模拟,然后进行分子力学泊松-玻尔兹曼表面积计算,以研究I-BRD9抑制剂H1B及其衍生物N1D,TVU和5V2对BRD9和BRD4的选择机理。我们计算的结合自由能的等级与实验数据的等级一致。结果表明,H1B与BRD7的结合自由能略低于H1B与BRD9的结合自由能,并且所有四种抑制剂与BRD9的结合比与BRD4的结合更紧密。结合自由能分解成单个残基意味着BRD7中的Ile164和Asn211以及BRD9中的Ile53和Asn100在H1B对BRD7和BRD9的选择性中起重要作用。除了,BRD9中的几个关键残基Phe44,Ile53,Asn100,Thr104和BRD4中的Pro82,Lys91,Asn140,Asp144位于ZA-loop和BC-loop中,这对抑制剂对BRD9和BRD4的结合选择性起了重要作用。预期该研究将为针对BRD9和BRD4的高选择性抑制剂的合理设计提供重要的理论指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号