当前位置:
X-MOL 学术
›
Eur. Polym. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interactions of Poly (vinyl alcohol) and Poly (N-isopropylacrylamide) with Tannic Acid and Pyrazole Derivatives: The Identification of H-Bonds for Revealing a “Two-Molecules” Carrier System
European Polymer Journal ( IF 6 ) Pub Date : 2018-11-01 , DOI: 10.1016/j.eurpolymj.2018.09.028 Salim Ok , Ahmet Altun
European Polymer Journal ( IF 6 ) Pub Date : 2018-11-01 , DOI: 10.1016/j.eurpolymj.2018.09.028 Salim Ok , Ahmet Altun
|
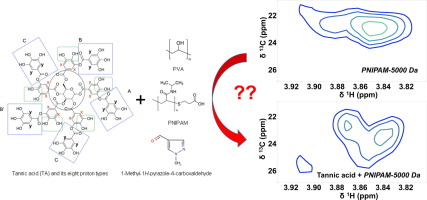
Abstract The interactions between tannic acid (TA), a natural bioactive polyphenol, and two biodegradable polymers have been investigated in the presence and absence of a pyrazole derivative through their NMR and UV–Vis spectra as well as quantum chemical calculations. The role of H-bonding ability of organic molecules is studied for clarifying the molecule carrier capability of the polymers. TA uses its hydrogen bonding capability controversially depending on the functional groups of the polymers in solutions/mixtures in DMSO. In the mixtures with poly (vinyl alcohol) (PVA), the color of TA is darkened, which associates to the appearance of a new UV–Vis band, the disappearance of two one-dimensional proton NMR signals, and some differences in the heteronuclear multiple bond correlation (HMBC) NMR spectra. The H-bond interactions in the mixtures of TA and PVA are mainly between oxygen of the polymer backbone and proton of OH of TA in addition to hydrogen bonding interaction between O atom of aromatic rings of TA and OH moiety of PVA as revealed by UV–Vis spectroscopy and computational results. Association of the pyrazole derivative in the mixture introduces a weak interaction between protons of OH belonging to outer core of TA and oxygen of C O of the pyrazole derivative. In the mixtures of TA with poly (N-isopropylacrylamide) (PNIPAM), a noticeable change is not observed in the UV–Vis and one-dimensional proton NMR spectra irrespective of the addition of the pyrazole derivative. However, the weak interactions between TA and the pyrazole derivative in mixtures with PNIPAM are apparent in their HMBC NMR spectra. PNIPAM and TA interact mainly through the proton of OH in the outer core of TA and the oxygen of C O of PNIPAM backbone as in the case of TA and the pyrazole derivative. They also interact through the C O in the central core of TA and proton of OH of PNIPAM backbone. To assess the effect of the different H-bond environments on the UV–Vis and NMR spectra, we have performed quantum chemical calculations employing B3LYP density functional approach. The present calculations appear quite useful in explaining the source of spectral differences in different media. Based on the spectral analysis, we suggest that PVA is a better molecule carrier with respect to PNIPAM.
中文翻译:

聚(乙烯醇)和聚(N-异丙基丙烯酰胺)与单宁酸和吡唑衍生物的相互作用:鉴定用于揭示“双分子”载体系统的氢键
摘要 单宁酸 (TA)、一种天然生物活性多酚和两种可生物降解的聚合物在吡唑衍生物存在和不存在的情况下,通过它们的 NMR 和 UV-Vis 光谱以及量子化学计算进行了研究。研究有机分子的氢键能力的作用,以阐明聚合物的分子载体能力。根据 DMSO 溶液/混合物中聚合物的官能团,TA 使用其氢键能力有争议。在与聚乙烯醇 (PVA) 的混合物中,TA 的颜色变暗,这与新的 UV-Vis 谱带的出现、两个一维质子 NMR 信号的消失以及异核的一些差异有关多键相关 (HMBC) NMR 光谱。TA 和 PVA 混合物中的氢键相互作用主要是在聚合物骨架的氧和 TA 的 OH 质子之间,除了 TA 的芳环的 O 原子和 PVA 的 OH 部分之间的氢键相互作用,如 UV-可见光谱和计算结果。混合物中吡唑衍生物的缔合在属于 TA 外核的 OH 质子与吡唑衍生物的 CO 氧之间引入了弱相互作用。在 TA 与聚(N-异丙基丙烯酰胺)(PNIPAM)的混合物中,无论是否添加吡唑衍生物,在 UV-Vis 和一维质子 NMR 光谱中都没有观察到明显的变化。然而,TA 和吡唑衍生物在与 PNIPAM 的混合物中的弱相互作用在它们的 HMBC NMR 光谱中很明显。PNIPAM 和 TA 主要通过 TA 外核中的 OH 质子和 PNIPAM 骨架的 CO 氧相互作用,如 TA 和吡唑衍生物的情况。它们还通过 TA 中心核心中的 CO 和 PNIPAM 主链的 OH 质子相互作用。为了评估不同氢键环境对 UV-Vis 和 NMR 光谱的影响,我们使用 B3LYP 密度泛函方法进行了量子化学计算。目前的计算在解释不同介质中光谱差异的来源方面似乎非常有用。基于光谱分析,我们认为 PVA 是比 PNIPAM 更好的分子载体。它们还通过 TA 中心核心中的 CO 和 PNIPAM 主链的 OH 质子相互作用。为了评估不同氢键环境对 UV-Vis 和 NMR 光谱的影响,我们使用 B3LYP 密度泛函方法进行了量子化学计算。目前的计算在解释不同介质中光谱差异的来源方面似乎非常有用。基于光谱分析,我们认为 PVA 是比 PNIPAM 更好的分子载体。它们还通过 TA 中心核心中的 CO 和 PNIPAM 主链的 OH 质子相互作用。为了评估不同氢键环境对 UV-Vis 和 NMR 光谱的影响,我们使用 B3LYP 密度泛函方法进行了量子化学计算。目前的计算在解释不同介质中光谱差异的来源方面似乎非常有用。基于光谱分析,我们认为 PVA 是比 PNIPAM 更好的分子载体。
更新日期:2018-11-01
中文翻译:

聚(乙烯醇)和聚(N-异丙基丙烯酰胺)与单宁酸和吡唑衍生物的相互作用:鉴定用于揭示“双分子”载体系统的氢键
摘要 单宁酸 (TA)、一种天然生物活性多酚和两种可生物降解的聚合物在吡唑衍生物存在和不存在的情况下,通过它们的 NMR 和 UV-Vis 光谱以及量子化学计算进行了研究。研究有机分子的氢键能力的作用,以阐明聚合物的分子载体能力。根据 DMSO 溶液/混合物中聚合物的官能团,TA 使用其氢键能力有争议。在与聚乙烯醇 (PVA) 的混合物中,TA 的颜色变暗,这与新的 UV-Vis 谱带的出现、两个一维质子 NMR 信号的消失以及异核的一些差异有关多键相关 (HMBC) NMR 光谱。TA 和 PVA 混合物中的氢键相互作用主要是在聚合物骨架的氧和 TA 的 OH 质子之间,除了 TA 的芳环的 O 原子和 PVA 的 OH 部分之间的氢键相互作用,如 UV-可见光谱和计算结果。混合物中吡唑衍生物的缔合在属于 TA 外核的 OH 质子与吡唑衍生物的 CO 氧之间引入了弱相互作用。在 TA 与聚(N-异丙基丙烯酰胺)(PNIPAM)的混合物中,无论是否添加吡唑衍生物,在 UV-Vis 和一维质子 NMR 光谱中都没有观察到明显的变化。然而,TA 和吡唑衍生物在与 PNIPAM 的混合物中的弱相互作用在它们的 HMBC NMR 光谱中很明显。PNIPAM 和 TA 主要通过 TA 外核中的 OH 质子和 PNIPAM 骨架的 CO 氧相互作用,如 TA 和吡唑衍生物的情况。它们还通过 TA 中心核心中的 CO 和 PNIPAM 主链的 OH 质子相互作用。为了评估不同氢键环境对 UV-Vis 和 NMR 光谱的影响,我们使用 B3LYP 密度泛函方法进行了量子化学计算。目前的计算在解释不同介质中光谱差异的来源方面似乎非常有用。基于光谱分析,我们认为 PVA 是比 PNIPAM 更好的分子载体。它们还通过 TA 中心核心中的 CO 和 PNIPAM 主链的 OH 质子相互作用。为了评估不同氢键环境对 UV-Vis 和 NMR 光谱的影响,我们使用 B3LYP 密度泛函方法进行了量子化学计算。目前的计算在解释不同介质中光谱差异的来源方面似乎非常有用。基于光谱分析,我们认为 PVA 是比 PNIPAM 更好的分子载体。它们还通过 TA 中心核心中的 CO 和 PNIPAM 主链的 OH 质子相互作用。为了评估不同氢键环境对 UV-Vis 和 NMR 光谱的影响,我们使用 B3LYP 密度泛函方法进行了量子化学计算。目前的计算在解释不同介质中光谱差异的来源方面似乎非常有用。基于光谱分析,我们认为 PVA 是比 PNIPAM 更好的分子载体。


























 京公网安备 11010802027423号
京公网安备 11010802027423号