当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel binaphthyl and biphenyl α- and β-amino acids and esters: organocatalysis of asymmetric Diels–Alder reactions. A combined synthetic and computational study
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-09-17 , DOI: 10.1039/c8ob01795f Philip C. Bulman Page 1, 2, 3, 4, 5 , Francesca S. Kinsey 1, 2, 3, 4, 5 , Yohan Chan 1, 2, 3, 4, 5 , Ian R. Strutt 1, 2, 3, 4, 5 , Alexandra M. Z. Slawin 1, 5, 6, 7 , Garth A. Jones 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-09-17 , DOI: 10.1039/c8ob01795f Philip C. Bulman Page 1, 2, 3, 4, 5 , Francesca S. Kinsey 1, 2, 3, 4, 5 , Yohan Chan 1, 2, 3, 4, 5 , Ian R. Strutt 1, 2, 3, 4, 5 , Alexandra M. Z. Slawin 1, 5, 6, 7 , Garth A. Jones 1, 2, 3, 4, 5
Affiliation
|
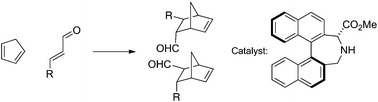
Asymmetric catalysis of the Diels–Alder reaction between cyclopentadiene and cinnamaldehydes has been studied using as catalysts a range of novel α- and β-aminoacids and aminoesters with binaphthyl and biphenyl backbones, providing enantioselectivities of up to 62% ee. B3LYP/6-31G* calculations, including free energy corrections, have been carried out on a binaphthyl catalyst example to identify transition state structures and to aid in the identification of major enantiomers. The calculated product ratios agree well with the experimental data; the transition states identified involve preferential approach of cyclopentene along a trajectory adjacent to the acid/ester group. The four lowest energy transition states display a stabilizing dipolar interaction between the carbonyl group oxygen atom and a terminal proton of the diene unit.
中文翻译:

新型联萘和联苯α-和β-氨基酸和酯:不对称Diels-Alder反应的有机催化。综合研究与计算研究
已经研究了环戊二烯与肉桂醛之间Diels-Alder反应的不对称催化,使用了一系列具有联萘和联苯骨架的新型α-和β-氨基酸和氨基酯,提供高达62%ee的对映选择性。已在联萘催化剂实例上进行了B3LYP / 6-31G *计算,包括自由能校正,以识别过渡态结构并帮助鉴定主要对映异构体。计算出的产品比例与实验数据非常吻合;所确定的过渡态涉及沿环戊烯沿酸/酯基团相邻的轨迹的优先途径。四个最低的能量跃迁状态显示出羰基氧原子与二烯单元的末端质子之间稳定的偶极相互作用。
更新日期:2018-10-18
中文翻译:

新型联萘和联苯α-和β-氨基酸和酯:不对称Diels-Alder反应的有机催化。综合研究与计算研究
已经研究了环戊二烯与肉桂醛之间Diels-Alder反应的不对称催化,使用了一系列具有联萘和联苯骨架的新型α-和β-氨基酸和氨基酯,提供高达62%ee的对映选择性。已在联萘催化剂实例上进行了B3LYP / 6-31G *计算,包括自由能校正,以识别过渡态结构并帮助鉴定主要对映异构体。计算出的产品比例与实验数据非常吻合;所确定的过渡态涉及沿环戊烯沿酸/酯基团相邻的轨迹的优先途径。四个最低的能量跃迁状态显示出羰基氧原子与二烯单元的末端质子之间稳定的偶极相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号