当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An In Situ Reversible Heterodimeric Nanoswitch Controlled by Metal‐Ion–Ligand Coordination Regulates the Mechanosensing and Differentiation of Stem Cells
Advanced Materials ( IF 29.4 ) Pub Date : 2018-09-16 , DOI: 10.1002/adma.201803591 Heemin Kang 1 , Kunyu Zhang 1 , Hee Joon Jung 2, 3, 4 , Boguang Yang 1 , Xiaoyu Chen 1 , Qi Pan 5 , Rui Li 1 , Xiayi Xu 1 , Gang Li 5, 6 , Vinayak P. Dravid 2, 3, 4 , Liming Bian 1, 6
Advanced Materials ( IF 29.4 ) Pub Date : 2018-09-16 , DOI: 10.1002/adma.201803591 Heemin Kang 1 , Kunyu Zhang 1 , Hee Joon Jung 2, 3, 4 , Boguang Yang 1 , Xiaoyu Chen 1 , Qi Pan 5 , Rui Li 1 , Xiayi Xu 1 , Gang Li 5, 6 , Vinayak P. Dravid 2, 3, 4 , Liming Bian 1, 6
Affiliation
|
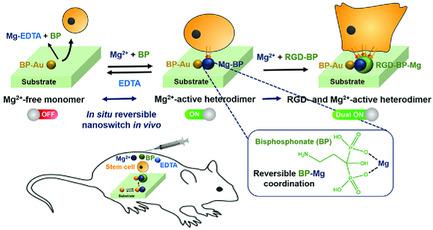
In situ and cytocompatible nanoswitching by external stimuli is highly appealing for reversibly regulating cellular adhesion and functions in vivo. Here, a heterodimeric nanoswitch is designed to facilitate in situ switchable and combinatorial presentation of integrin‐binding cell‐adhesive moieties, such as Mg2+ and Arg‐Gly‐Asp (RGD) ligand in nanostructures. In situ reversible nanoswitching is controlled by convertible coordination between bioactive Mg2+ and bisphosphonate (BP) ligand. A BP‐coated gold‐nanoparticle monomer (BP‐AuNP) on a substrate is prepared to allow in situ assembly of cell‐adhesive Mg2+‐active Mg‐BP nanoparticles (NPs) on a BP‐AuNP surface via Mg2+‐BP coordination, yielding heterodimeric nanostructures (switching “ON”). Ethylenediaminetetraacetic acid (EDTA)‐based Mg2+ chelation allows in situ disassembly of Mg2+‐BP NP, reverting to Mg2+‐free monomer (switching “OFF”). This in situ reversible nanoswitching on and off of cell‐adhesive Mg2+ presentation allows reversible cell adhesion and release in vivo, respectively, and spatiotemporally controls cyclic cell adhesion. In situ heterodimeric assembly of dual RGD ligand‐ and Mg2+‐active RGD‐BP‐Mg2+ NP (switching “Dual ON”) further tunes and promotes focal adhesion, spreading, and differentiation of stem cells. The modular nature of this in situ nanoswitch can accommodate various bioactive nanostructures via metal‐ion–ligand coordination to regulate diverse cellular functions in vivo in reversible and compatible manner.
中文翻译:

金属离子配体控制的原位可逆异二聚纳米开关调节干细胞的机械传感和分化。
外在刺激的原位和细胞相容性纳米开关对于可逆地调节体内的细胞黏附和功能非常有吸引力。在这里,设计了一个异二聚体纳米开关,以促进原位结合蛋白结合细胞粘附部分的原位可转换和组合表达,例如纳米结构中的Mg 2+和Arg-Gly-Asp(RGD)配体。原位可逆的纳米转换是由生物活性Mg 2+和双膦酸酯(BP)配体之间的可转换配位控制的。准备在基材上涂有BP涂层的金纳米颗粒单体(BP-AuNP),以通过Mg 2+在BP-AuNP表面上原位组装具有细胞粘附性的Mg 2 + -活性Mg-BP纳米颗粒(NPs)。‐BP配位,产生异二聚体纳米结构(切换为“ ON”)。基于乙二胺四乙酸(EDTA)的Mg 2+螯合剂可原位分解Mg 2+ -BP NP,恢复为不含Mg 2+的单体(切换为“ OFF”)。这种Mg 2+粘附在细胞表面的可逆的纳米开关可分别使细胞在体内发生可逆的粘附和释放,并时空控制循环细胞的粘附。双RGD配体和Mg 2+活性RGD-BP-Mg 2+的原位异二聚体组装NP(将“ Dual ON”切换为“ Dual ON”)可进一步调节并促进干细胞的粘着,扩散和分化。这种原位纳米开关的模块化性质可以通过金属离子-配体的配合来适应各种生物活性纳米结构,从而以可逆和兼容的方式调节体内多种细胞功能。
更新日期:2018-09-16
中文翻译:

金属离子配体控制的原位可逆异二聚纳米开关调节干细胞的机械传感和分化。
外在刺激的原位和细胞相容性纳米开关对于可逆地调节体内的细胞黏附和功能非常有吸引力。在这里,设计了一个异二聚体纳米开关,以促进原位结合蛋白结合细胞粘附部分的原位可转换和组合表达,例如纳米结构中的Mg 2+和Arg-Gly-Asp(RGD)配体。原位可逆的纳米转换是由生物活性Mg 2+和双膦酸酯(BP)配体之间的可转换配位控制的。准备在基材上涂有BP涂层的金纳米颗粒单体(BP-AuNP),以通过Mg 2+在BP-AuNP表面上原位组装具有细胞粘附性的Mg 2 + -活性Mg-BP纳米颗粒(NPs)。‐BP配位,产生异二聚体纳米结构(切换为“ ON”)。基于乙二胺四乙酸(EDTA)的Mg 2+螯合剂可原位分解Mg 2+ -BP NP,恢复为不含Mg 2+的单体(切换为“ OFF”)。这种Mg 2+粘附在细胞表面的可逆的纳米开关可分别使细胞在体内发生可逆的粘附和释放,并时空控制循环细胞的粘附。双RGD配体和Mg 2+活性RGD-BP-Mg 2+的原位异二聚体组装NP(将“ Dual ON”切换为“ Dual ON”)可进一步调节并促进干细胞的粘着,扩散和分化。这种原位纳米开关的模块化性质可以通过金属离子-配体的配合来适应各种生物活性纳米结构,从而以可逆和兼容的方式调节体内多种细胞功能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号