当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Novel Derivatives of 1,2,4‐Triazoles
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-09-17 , DOI: 10.1002/slct.201802243 Armen S. Galstyan 1 , Tariel V. Ghochikyan 1 , Vardges R. Frangyan 1 , Rafael A. Tamazyan 2 , Armen G. Ayvazyan 2
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-09-17 , DOI: 10.1002/slct.201802243 Armen S. Galstyan 1 , Tariel V. Ghochikyan 1 , Vardges R. Frangyan 1 , Rafael A. Tamazyan 2 , Armen G. Ayvazyan 2
Affiliation
|
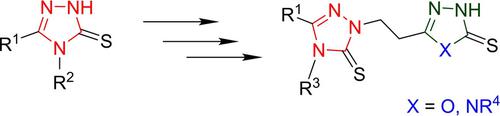
A method for the preparation of methyl esters of β‐1,2,4‐triazole‐substituted alanines by condensation of methyl acrylate with 3,4‐disubstituted‐5‐mercapto‐1,2,4‐triazoles has been developed. The optimal conditions for the process of hydrazinolysis of N‐allyl derivatives of the obtained products were established. It is shown that to prevent the reduction of the allyl group, it is reasonable to carry out the process of hydrazinolysis in the presence of sodium sulfite. Based on hydrazides of β‐1,2,4‐triazole‐substituted alanines, biheterocyclic compounds of a new structure – 1,2,4‐triazole‐ and 1,3,4‐oxadiazole‐1,2,4‐triazoles were synthesized.
中文翻译:

1,2,4-三唑新型衍生物的合成
已开发出一种通过丙烯酸甲酯与3,4-二取代-5-巯基1,2,4-三唑缩合制备β-1,2,4-三唑取代的丙氨酸甲酯的方法。确定了获得的产物的N-烯丙基衍生物进行肼解反应的最佳条件。结果表明,为了防止烯丙基的还原,在亚硫酸钠的存在下进行肼解反应是合理的。基于β1,2,4-三唑取代的丙氨酸的酰肼,合成了新结构的双杂环化合物–1,2,4-三唑和1,3,4-恶二唑-1,2,4-三唑。
更新日期:2018-09-17
中文翻译:

1,2,4-三唑新型衍生物的合成
已开发出一种通过丙烯酸甲酯与3,4-二取代-5-巯基1,2,4-三唑缩合制备β-1,2,4-三唑取代的丙氨酸甲酯的方法。确定了获得的产物的N-烯丙基衍生物进行肼解反应的最佳条件。结果表明,为了防止烯丙基的还原,在亚硫酸钠的存在下进行肼解反应是合理的。基于β1,2,4-三唑取代的丙氨酸的酰肼,合成了新结构的双杂环化合物–1,2,4-三唑和1,3,4-恶二唑-1,2,4-三唑。


























 京公网安备 11010802027423号
京公网安备 11010802027423号