当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Iminyl Radical‐Mediated Controlled Hydroxyalkylation of Remote C(sp3)‐H Bond via Tandem 1,5‐HAT and Difunctionalization of Aryl Alkenes
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-10-11 , DOI: 10.1002/adsc.201801198 Zhi-Yong Ma 1 , Li-Na Guo 1 , Yu-Rui Gu 1 , Li Chen 1 , Xin-Hua Duan 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-10-11 , DOI: 10.1002/adsc.201801198 Zhi-Yong Ma 1 , Li-Na Guo 1 , Yu-Rui Gu 1 , Li Chen 1 , Xin-Hua Duan 1
Affiliation
|
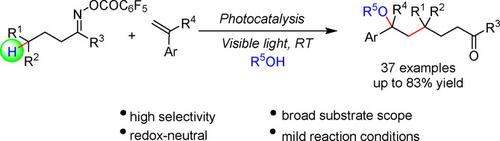
A visible‐light mediated γ‐hydroxyalkylation of ketones via C(sp3)‐H functionalization has been developed under redox neutral conditions. This protocol relies on the iminyl radical‐triggered 1,5‐HAT followed by oxyalkylation of alkenes, wherein C−C and C−O bonds were constructed in one step. This three‐component reaction features mild conditions, wide substrate scope and excellent functional group tolerance, thus providing a facile and highly efficient access to complex valuable ketones.
中文翻译:

通过串联1,5-HAT的远程C(sp3)-H键的亚氨基自由基介导的受控羟烷基化和芳基烯烃的双官能化
在氧化还原中性条件下,通过C(sp 3)-H官能团形成了可见光介导的酮的γ-羟烷基化反应。该协议依赖于亚胺基引发的1,5-HAT,然后进行烯烃的氧烷基化,其中CC和C-O键是一步构建的。该三组分反应具有温和的条件,宽的底物范围和出色的官能团耐受性,因此可以轻松高效地获得复杂的有价值的酮。
更新日期:2018-10-11
中文翻译:

通过串联1,5-HAT的远程C(sp3)-H键的亚氨基自由基介导的受控羟烷基化和芳基烯烃的双官能化
在氧化还原中性条件下,通过C(sp 3)-H官能团形成了可见光介导的酮的γ-羟烷基化反应。该协议依赖于亚胺基引发的1,5-HAT,然后进行烯烃的氧烷基化,其中CC和C-O键是一步构建的。该三组分反应具有温和的条件,宽的底物范围和出色的官能团耐受性,因此可以轻松高效地获得复杂的有价值的酮。



























 京公网安备 11010802027423号
京公网安备 11010802027423号