当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Self-Supported Hydrous Iridium–Nickel Oxide Two-Dimensional Nanoframes for High Activity Oxygen Evolution Electrocatalysts
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-09-14 00:00:00 , DOI: 10.1021/acscatal.8b02171 Fernando Godínez-Salomón 1 , Luis Albiter 1 , Shaun M. Alia 2 , Bryan S. Pivovar 2 , Luis E. Camacho-Forero 3 , Perla B. Balbuena 3 , Rubén Mendoza-Cruz 4 , M. Josefina Arellano-Jimenez 4 , Christopher P. Rhodes 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-09-14 00:00:00 , DOI: 10.1021/acscatal.8b02171 Fernando Godínez-Salomón 1 , Luis Albiter 1 , Shaun M. Alia 2 , Bryan S. Pivovar 2 , Luis E. Camacho-Forero 3 , Perla B. Balbuena 3 , Rubén Mendoza-Cruz 4 , M. Josefina Arellano-Jimenez 4 , Christopher P. Rhodes 1
Affiliation
|
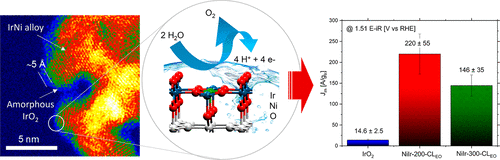
Oxygen evolution reaction (OER) electrocatalysts with high activity, high stability, and low costs are needed for proton-exchange membrane (PEM) electrolyzers. Based on the high cost and limited supply of iridium, approaches that result in iridium-based OER catalysts with increased catalytic activity are of significant interest. We report a carbon-free, self-supported hydrous iridium–nickel oxide two-dimensional nanoframe structure synthesized by thermal treatment of iridium-decorated nickel oxide nanosheets under reducing conditions and subsequent chemical leaching in acid. The catalyst nanoarchitecture contains an interconnected network of metallic iridium–nickel alloy domains with hydrous iridium oxide and nickel oxide located in the surface region. The electrochemical oxidation step maintains the three-dimensional nanoarchitecture and results in a thin (∼5 Å) oxide/hydroxide surface layer. The temperature used for thermal reduction was found to strongly affect the catalyst surface structure and OER activity. Using a lower thermal reduction temperature of 200 °C was determined to provide a higher relative surface concentration of hydroxides and nickel oxide and result in higher OER activities compared with materials treated at 300 °C. The 200 °C-treated hydrous iridium–nickel oxide electrocatalyst showed 15 times higher initial OER mass activity than commercial IrO2, and the activity remained 10 times higher than IrO2 after accelerated durability testing. Density functional theory (DFT) calculations and analysis of the experimental Tafel slopes support that the second electron transfer step is the rate-limiting step for the reaction. The DFT calculations demonstrate that Ni substitution on the IrO2 surface lowers the activation energy for adsorbed intermediates of the second electron transfer step of the OER reaction. This work establishes that noble metal-decorated metal oxide nanosheets can be transformed into high surface area, carbon-free electrocatalyst nanostructures with high catalytic activities and molecular accessibility and reveals the importance of using controlled thermal reduction temperatures to alter the surface structure and OER activity.
中文翻译:

高活性氧析出电催化剂的自支撑含水铱-氧化镍二维纳米框架
质子交换膜(PEM)电解槽需要具有高活性,高稳定性和低成本的氧析出反应(OER)电催化剂。基于铱的高成本和有限的供应,导致具有提高的催化活性的基于铱的OER催化剂的方法引起了人们的极大兴趣。我们报告了一种无碳,自支撑的含水铱-氧化镍二维纳米框架结构,该结构是通过在还原条件下对铱装饰的氧化镍纳米片进行热处理并随后在酸中进行化学浸出而合成的。催化剂纳米结构包含金属铱-镍合金域的互连网络,在表面区域中存在含水的氧化铱和氧化镍。电化学氧化步骤可维持三维纳米结构,并形成薄的(〜5Å)氧化物/氢氧化物表面层。发现用于热还原的温度强烈影响催化剂的表面结构和OER活性。与在300°C下处理的材料相比,确定使用200°C的较低的热还原温度可提供较高的氢氧化物和氧化镍的相对表面浓度,并导致较高的OER活性。经200°C处理的含水铱-氧化镍电催化剂显示的初始OER质量活性是商业IrO的15倍 与在300°C下处理的材料相比,确定使用200°C的较低的热还原温度可提供较高的氢氧化物和氧化镍的相对表面浓度,并导致较高的OER活性。经200°C处理的含水铱-氧化镍电催化剂显示的初始OER质量活性是商业IrO的15倍 与在300°C下处理的材料相比,确定使用200°C的较低的热还原温度可提供较高的氢氧化物和氧化镍的相对表面浓度,并导致较高的OER活性。经200°C处理的含水铱-氧化镍电催化剂显示的初始OER质量活性是商业IrO的15倍如图2所示,在加速耐久性试验后,活性仍然是IrO 2的10倍。密度泛函理论(DFT)的计算和实验塔菲尔斜率的分析支持第二电子转移步骤是反应的限速步骤。DFT计算表明,IrO 2上的镍取代表面降低了OER反应第二电子转移步骤中吸附的中间体的活化能。这项工作建立了贵金属装饰的金属氧化物纳米片可以转变为具有高催化活性和分子可及性的高表面积,无碳的电催化剂纳米结构,并揭示了使用受控的热还原温度来改变表面结构和OER活性的重要性。
更新日期:2018-09-14
中文翻译:

高活性氧析出电催化剂的自支撑含水铱-氧化镍二维纳米框架
质子交换膜(PEM)电解槽需要具有高活性,高稳定性和低成本的氧析出反应(OER)电催化剂。基于铱的高成本和有限的供应,导致具有提高的催化活性的基于铱的OER催化剂的方法引起了人们的极大兴趣。我们报告了一种无碳,自支撑的含水铱-氧化镍二维纳米框架结构,该结构是通过在还原条件下对铱装饰的氧化镍纳米片进行热处理并随后在酸中进行化学浸出而合成的。催化剂纳米结构包含金属铱-镍合金域的互连网络,在表面区域中存在含水的氧化铱和氧化镍。电化学氧化步骤可维持三维纳米结构,并形成薄的(〜5Å)氧化物/氢氧化物表面层。发现用于热还原的温度强烈影响催化剂的表面结构和OER活性。与在300°C下处理的材料相比,确定使用200°C的较低的热还原温度可提供较高的氢氧化物和氧化镍的相对表面浓度,并导致较高的OER活性。经200°C处理的含水铱-氧化镍电催化剂显示的初始OER质量活性是商业IrO的15倍 与在300°C下处理的材料相比,确定使用200°C的较低的热还原温度可提供较高的氢氧化物和氧化镍的相对表面浓度,并导致较高的OER活性。经200°C处理的含水铱-氧化镍电催化剂显示的初始OER质量活性是商业IrO的15倍 与在300°C下处理的材料相比,确定使用200°C的较低的热还原温度可提供较高的氢氧化物和氧化镍的相对表面浓度,并导致较高的OER活性。经200°C处理的含水铱-氧化镍电催化剂显示的初始OER质量活性是商业IrO的15倍如图2所示,在加速耐久性试验后,活性仍然是IrO 2的10倍。密度泛函理论(DFT)的计算和实验塔菲尔斜率的分析支持第二电子转移步骤是反应的限速步骤。DFT计算表明,IrO 2上的镍取代表面降低了OER反应第二电子转移步骤中吸附的中间体的活化能。这项工作建立了贵金属装饰的金属氧化物纳米片可以转变为具有高催化活性和分子可及性的高表面积,无碳的电催化剂纳米结构,并揭示了使用受控的热还原温度来改变表面结构和OER活性的重要性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号