Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oxidative Deposition of Manganese Oxide Nanosheets on Nitrogen-Functionalized Carbon Nanotubes Applied in the Alkaline Oxygen Evolution Reaction
ACS Omega ( IF 4.1 ) Pub Date : 2018-09-14 00:00:00 , DOI: 10.1021/acsomega.8b01433 Hendrik Antoni , Dulce M. Morales , Qi Fu , Yen-Ting Chen , Justus Masa , Wolfgang Schuhmann , Martin Muhler
ACS Omega ( IF 4.1 ) Pub Date : 2018-09-14 00:00:00 , DOI: 10.1021/acsomega.8b01433 Hendrik Antoni , Dulce M. Morales , Qi Fu , Yen-Ting Chen , Justus Masa , Wolfgang Schuhmann , Martin Muhler
|
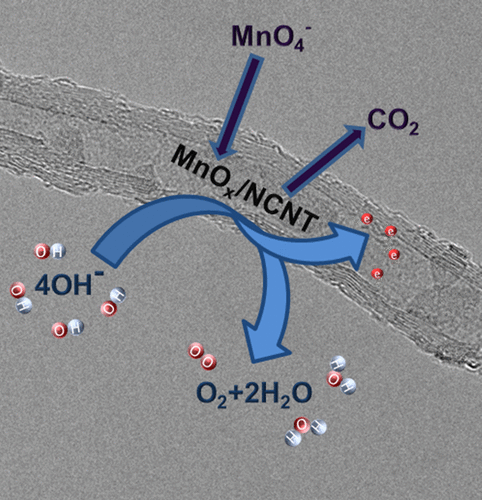
The development of nonprecious catalysts for water splitting into hydrogen and oxygen is one of the major challenges to meet future sustainable fuel demand. Herein, thin layers of manganese oxide nanosheets supported on nitrogen-functionalized carbon nanotubes (NCNTs) were formed by the treatment of NCNTs dispersed in aqueous solutions of KMnO4 or CsMnO4 under reflux or under hydrothermal (HT) conditions and used as electrocatalysts for the oxygen evolution reaction (OER) in alkaline media. The samples were characterized by X-ray photoelectron spectroscopy, X-ray diffraction, transmission electron microscopy, and Raman spectroscopy. Our results show that the NCNTs treated under reflux were covered by partly amorphous and birnessite-type manganese oxides, while predominantly crystalline birnessite manganese oxide was observed for the hydrothermally treated samples. The latter showed, depending on the temperature during synthesis, an electrocatalytically favorable reduction from birnessite-type MnO2 to γ-MnOOH. OER activity measurements revealed a decrease of the overpotential for the OER at a current density of 10 mA cm–2 from 1.70 VRHE for the bare NCNTs to 1.64 VRHE for the samples treated under reflux in the presence of KMnO4. The hydrothermally treated samples afforded the same current density at a lower potential of 1.60 VRHE and a Tafel slope of 75 mV dec–1, suggesting that the higher OER activity is due to γ-MnOOH formation. Oxidative deposition under reflux conditions using CsMnO4 along with mild HT treatment using KMnO4, and low manganese loadings in both cases, were identified as the most suitable synthetic routes to obtain highly active MnOx/NCNT catalysts for electrochemical water oxidation.
中文翻译:

氮官能化碳纳米管在碱性氧析出反应中的氧化锰纳米片氧化沉积
开发用于将水分解为氢气和氧气的非贵重催化剂是满足未来可持续燃料需求的主要挑战之一。在此,通过分散在KMnO 4或CsMnO 4的水溶液中的NCNT的处理,形成支撑在氮官能化的碳纳米管(NCNT)上的氧化锰纳米片的薄层。在回流或水热(HT)条件下,并用作碱性介质中氧释放反应(OER)的电催化剂。通过X射线光电子能谱,X射线衍射,透射电子显微镜和拉曼光谱对样品进行表征。我们的结果表明,在回流条件下处理的NCNT被非晶态和水钠锰矿型锰氧化物部分覆盖,而在水热处理后的样品中主要观察到结晶水钠锰矿型锰氧化物。根据合成过程中的温度,后者显示出从水钠锰矿型MnO 2到γ-MnOOH的电催化有利还原。OER活性测量表明,在1.70 V的电流密度下,电流密度为10 mA cm –2时,OER的过电势降低了RHE为裸NCNTs到1.64 V RHE在高锰酸钾存在下回流处理过的样品4。经过水热处理的样品在1.60 V RHE的较低电势和75 mV dec -1的Tafel斜率下提供了相同的电流密度,这表明较高的OER活性归因于γ-MnOOH的形成。在这两种情况下,使用CsMnO 4在回流条件下进行氧化沉积以及使用KMnO 4进行温和的HT处理以及低锰含量被认为是获得用于电化学水氧化的高活性MnO x / NCNT催化剂的最合适的合成途径。
更新日期:2018-09-14
中文翻译:

氮官能化碳纳米管在碱性氧析出反应中的氧化锰纳米片氧化沉积
开发用于将水分解为氢气和氧气的非贵重催化剂是满足未来可持续燃料需求的主要挑战之一。在此,通过分散在KMnO 4或CsMnO 4的水溶液中的NCNT的处理,形成支撑在氮官能化的碳纳米管(NCNT)上的氧化锰纳米片的薄层。在回流或水热(HT)条件下,并用作碱性介质中氧释放反应(OER)的电催化剂。通过X射线光电子能谱,X射线衍射,透射电子显微镜和拉曼光谱对样品进行表征。我们的结果表明,在回流条件下处理的NCNT被非晶态和水钠锰矿型锰氧化物部分覆盖,而在水热处理后的样品中主要观察到结晶水钠锰矿型锰氧化物。根据合成过程中的温度,后者显示出从水钠锰矿型MnO 2到γ-MnOOH的电催化有利还原。OER活性测量表明,在1.70 V的电流密度下,电流密度为10 mA cm –2时,OER的过电势降低了RHE为裸NCNTs到1.64 V RHE在高锰酸钾存在下回流处理过的样品4。经过水热处理的样品在1.60 V RHE的较低电势和75 mV dec -1的Tafel斜率下提供了相同的电流密度,这表明较高的OER活性归因于γ-MnOOH的形成。在这两种情况下,使用CsMnO 4在回流条件下进行氧化沉积以及使用KMnO 4进行温和的HT处理以及低锰含量被认为是获得用于电化学水氧化的高活性MnO x / NCNT催化剂的最合适的合成途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号