当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Water‐Triggered Metal‐Free Synthesis of Pyranopyrazoles via One‐Pot Oxidation/Michael Addition/Cyclization/Dehydration Sequence.
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-09-12 , DOI: 10.1002/slct.201801691 Anshu Dandia 1 , Sarika Bansal 1 , Ruchi Sharma 1 , Vijay Parewa 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-09-12 , DOI: 10.1002/slct.201801691 Anshu Dandia 1 , Sarika Bansal 1 , Ruchi Sharma 1 , Vijay Parewa 1
Affiliation
|
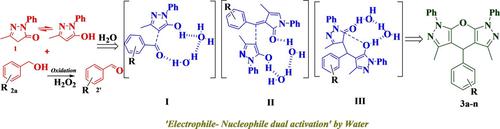
An “all water” strategy for the catalyst free chemo‐selective synthesis of pyranopyrazoles via the reaction of 3‐methyl‐1‐phenyl‐1H‐pyrazol‐5(4H)‐one with benzyl alcohols has been developed. Water actuates the reaction via ‘electrophile– nucleophile dual activation’ of the reactants through the concerted hydrogen bonding network and impel the reactants for C−C bond formation. Reaction proceeds by metal and catalyst free oxidation–Michael addition–cyclization–dehydration sequence. This method gave pyrano[2, 3‐c:6,5‐c′]dipyrazol]‐2‐ones selectivity over other possible products. Furthermore, this method is also applied on the reaction of the benzyl alcohols and dimedone to give arylmethylene[bis(5,5‐dimethyl‐3‐hydroxy‐2‐cyclohexene‐1‐ones)] in good to excellent yields.
中文翻译:

通过一锅氧化/迈克尔加成/环化/脱水序列进行水引发的吡喃并吡咯的无金属合成。
已开发出一种“全水”策略,用于通过3-甲基-1-苯基-1H-吡唑-5(4 H)-one与苄醇的无催化剂化学选择性合成吡喃并吡唑。水通过以下方式驱动反应反应物通过协调的氢键网络“亲电-亲核双重活化”,并推动反应物形成CC键。通过金属和催化剂的自由氧化-迈克尔加成-环化-脱水顺序进行反应。该方法提供了吡喃并[2,3-c:6,5-c']双吡唑] -2-酮对其他可能产物的选择性。此外,该方法还用于苯甲醇与二甲酮的反应,以良好的收率获得了芳基亚甲基[双(5,5-二甲基-3-羟基-2-环己烯-1-酮)]。
更新日期:2018-09-12
中文翻译:

通过一锅氧化/迈克尔加成/环化/脱水序列进行水引发的吡喃并吡咯的无金属合成。
已开发出一种“全水”策略,用于通过3-甲基-1-苯基-1H-吡唑-5(4 H)-one与苄醇的无催化剂化学选择性合成吡喃并吡唑。水通过以下方式驱动反应反应物通过协调的氢键网络“亲电-亲核双重活化”,并推动反应物形成CC键。通过金属和催化剂的自由氧化-迈克尔加成-环化-脱水顺序进行反应。该方法提供了吡喃并[2,3-c:6,5-c']双吡唑] -2-酮对其他可能产物的选择性。此外,该方法还用于苯甲醇与二甲酮的反应,以良好的收率获得了芳基亚甲基[双(5,5-二甲基-3-羟基-2-环己烯-1-酮)]。



























 京公网安备 11010802027423号
京公网安备 11010802027423号