当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insight into wild-type and T1372E TET2-mediated 5hmC oxidation using ab initio QM/MM calculations†
Chemical Science ( IF 8.4 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1039/c8sc02961j Hedieh Torabifard 1 , G Andrés Cisneros 2
Chemical Science ( IF 8.4 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1039/c8sc02961j Hedieh Torabifard 1 , G Andrés Cisneros 2
Affiliation
|
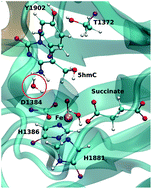
Ten-eleven translocation 2 (TET2) is an Fe/α-ketoglutarate (α-KG) dependent enzyme that dealkylates 5-methylcytosine (5mC). The reaction mechanism involves a series of three sequential oxidations that convert 5mC to 5-hydroxy-methylcytosine (5hmC), 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC). Our previous biochemical and computational studies uncovered an active site scaffold that is required for wild-type (WT) stepwise oxidation (Nat. Chem. Bio., 13, 181). We showed that the mutation of a single residue, T1372 to some amino acids, such as Glu, can impact the iterative oxidation steps and stop the oxidation of 5hmC to 5fC/caC. However, the source of the stalling at the first oxidation step by some mutant TET proteins still remains unclear. Here, we studied the catalytic mechanism of oxidation of 5hmC to 5fC by WT and T1372E TET2 using an ab initio quantum mechanical/molecular mechanical (QM/MM) approach. Our results suggest that the rate limiting step for WT TET2 involves a hydrogen atom abstraction from the hydroxyl group of 5hmC by the ferryl moiety in the WT. By contrast, our calculations for the T1372E mutant indicate that the rate limiting step for this variant corresponds to a second proton abstraction and the calculated barrier is almost twice as large as for WT TET2. Our results suggest that the large barrier for the 5hmC to 5fC oxidation in this mutant is due (at least in part) to the unfavorable orientation of the substrate in the active site. Combined electron localization function (ELF) and non-covalent interaction (NCI) analyses provide a qualitative description of the evolution of the electronic structure of the active site along the reaction path. Energy decomposition analysis (EDA) has been performed on the WT to investigate the impact of each MM residue on catalytic activity.
中文翻译:

使用从头开始 QM/MM 计算深入了解野生型和 T1372E TET2 介导的 5hmC 氧化†
10-11 易位 2 (TET2) 是一种 Fe/α-酮戊二酸 (α-KG) 依赖性酶,可使 5-甲基胞嘧啶 (5mC) 脱烷基化。反应机制涉及一系列三个连续氧化,将 5mC 转化为 5-羟甲基胞嘧啶 (5hmC)、5-甲酰胞嘧啶 (5fC) 和 5-羧基胞嘧啶 (5caC)。我们之前的生化和计算研究发现了野生型 (WT) 逐步氧化所需的活性位点支架(Nat. Chem. Bio. , 13 , 181)。我们发现,单个残基 T1372 突变为某些氨基酸(例如 Glu)可以影响迭代氧化步骤并阻止 5hmC 氧化为 5fC/caC。然而,一些突变 TET 蛋白在第一步氧化步骤中停滞的来源仍不清楚。在这里,我们使用从头算量子力学/分子力学 (QM/MM) 方法研究了 WT 和 T1372E TET2 将 5hmC 氧化为 5fC 的催化机制。我们的结果表明,WT TET2 的限速步骤涉及 WT 中的 ferr1 部分从 5hmC 的羟基中夺取氢原子。相比之下,我们对 T1372E 突变体的计算表明,该变体的速率限制步骤对应于第二次质子提取,并且计算出的势垒几乎是 WT TET2 的两倍。我们的结果表明,该突变体中 5hmC 至 5fC 氧化的巨大障碍是由于(至少部分)由于活性位点中底物的不利方向。电子定位函数 (ELF) 和非共价相互作用 (NCI) 组合分析提供了活性位点电子结构沿反应路径演化的定性描述。对 WT 进行了能量分解分析 (EDA),以研究每个 MM 残基对催化活性的影响。
更新日期:2018-09-11
中文翻译:

使用从头开始 QM/MM 计算深入了解野生型和 T1372E TET2 介导的 5hmC 氧化†
10-11 易位 2 (TET2) 是一种 Fe/α-酮戊二酸 (α-KG) 依赖性酶,可使 5-甲基胞嘧啶 (5mC) 脱烷基化。反应机制涉及一系列三个连续氧化,将 5mC 转化为 5-羟甲基胞嘧啶 (5hmC)、5-甲酰胞嘧啶 (5fC) 和 5-羧基胞嘧啶 (5caC)。我们之前的生化和计算研究发现了野生型 (WT) 逐步氧化所需的活性位点支架(Nat. Chem. Bio. , 13 , 181)。我们发现,单个残基 T1372 突变为某些氨基酸(例如 Glu)可以影响迭代氧化步骤并阻止 5hmC 氧化为 5fC/caC。然而,一些突变 TET 蛋白在第一步氧化步骤中停滞的来源仍不清楚。在这里,我们使用从头算量子力学/分子力学 (QM/MM) 方法研究了 WT 和 T1372E TET2 将 5hmC 氧化为 5fC 的催化机制。我们的结果表明,WT TET2 的限速步骤涉及 WT 中的 ferr1 部分从 5hmC 的羟基中夺取氢原子。相比之下,我们对 T1372E 突变体的计算表明,该变体的速率限制步骤对应于第二次质子提取,并且计算出的势垒几乎是 WT TET2 的两倍。我们的结果表明,该突变体中 5hmC 至 5fC 氧化的巨大障碍是由于(至少部分)由于活性位点中底物的不利方向。电子定位函数 (ELF) 和非共价相互作用 (NCI) 组合分析提供了活性位点电子结构沿反应路径演化的定性描述。对 WT 进行了能量分解分析 (EDA),以研究每个 MM 残基对催化活性的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号