当前位置:
X-MOL 学术
›
Nanoscale Horiz.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nanostructure of the deep eutectic solvent/platinum electrode interface as a function of potential and water content†
Nanoscale Horizons ( IF 9.7 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1039/c8nh00272j Oliver S. Hammond 1, 2, 3, 4 , Hua Li 5, 6, 7, 8 , Christian Westermann 9, 10, 11, 12 , Azhar Y. M. Al-Murshedi 4, 13, 14, 15, 16 , Frank Endres 9, 10, 11, 12 , Andrew P. Abbott 4, 13, 14, 15, 16 , Gregory G. Warr 8, 17, 18, 19 , Karen J. Edler 1, 2, 3, 4 , Rob Atkin 5, 6, 7, 8
Nanoscale Horizons ( IF 9.7 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1039/c8nh00272j Oliver S. Hammond 1, 2, 3, 4 , Hua Li 5, 6, 7, 8 , Christian Westermann 9, 10, 11, 12 , Azhar Y. M. Al-Murshedi 4, 13, 14, 15, 16 , Frank Endres 9, 10, 11, 12 , Andrew P. Abbott 4, 13, 14, 15, 16 , Gregory G. Warr 8, 17, 18, 19 , Karen J. Edler 1, 2, 3, 4 , Rob Atkin 5, 6, 7, 8
Affiliation
|
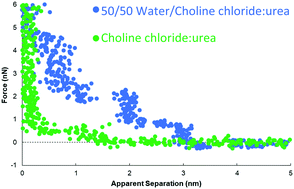
The interfacial nanostructure of the three most widely-studied Deep Eutectic Solvents (DESs), choline chloride:urea (ChCl:Urea), choline chloride:ethylene glycol (ChCl:EG), and choline chloride:glycerol (ChCl:Gly) at a Pt(111) electrode has been studied as a function of applied potential and water content up to 50 wt%. Contact mode atomic force microscope (AFM) force–distance curves reveal that for all three DESs, addition of water increases the interfacial nanostructure up to ∼40 wt%, after which it decreases. This differs starkly from ionic liquids, where addition of small amounts of water rapidly decreases the interfacial nanostructure. For the pure DESs, only one interfacial layer is measured at OCP at 0.5 nm, which increases to 3 to 6 layers extending ∼5 nm from the surface at 40 or 50 wt% water. Application of a potential of ±0.25 V to the Pt electrode for the pure DESs increases the number of near surface layers to 3. However, when water is present the applied potential attenuates the steps in the force curve, which are replaced by a short-range exponential decay. This change was most pronounced for ChCl:EG with 30 wt% or 50 wt% water, so this system was probed using cyclic voltammetry, which confirms the interfacial nanostructure is akin to a salt solution.
中文翻译:

低共熔溶剂/铂电极界面的纳米结构与电势和水含量的关系†
研究最多的三种深共晶溶剂(DESs),氯化胆碱:尿素(ChCl:尿素),氯化胆碱:乙二醇(ChCl:EG)和氯化胆碱:甘油(ChCl:Gly)的界面纳米结构已经研究了Pt(111)电极与施加电势和水含量不超过50 wt%的函数。接触模式原子力显微镜(AFM)的力-距离曲线显示,对于所有三个DES,添加水可使界面纳米结构增加至约40 wt%,然后降低。这与离子液体完全不同,在离子液体中添加少量水会迅速降低界面纳米结构。对于纯DES,在OCP处在0.5 nm处仅测量到一个界面层,在40或50 wt%的水处,该界面层增加至从表面延伸约5 nm的3至6层。施加±0的电势。对于纯DES,向Pt电极施加25 V电压会使近表面层的数量增加至3。但是,当存在水时,施加的电势会削弱力曲线中的阶跃,并由短程指数衰减代替。对于含30 wt%或50 wt%的水的ChCl:EG,这种变化最为明显,因此使用循环伏安法对该系统进行了探测,这证实了界面纳米结构类似于盐溶液。
更新日期:2018-09-11
中文翻译:

低共熔溶剂/铂电极界面的纳米结构与电势和水含量的关系†
研究最多的三种深共晶溶剂(DESs),氯化胆碱:尿素(ChCl:尿素),氯化胆碱:乙二醇(ChCl:EG)和氯化胆碱:甘油(ChCl:Gly)的界面纳米结构已经研究了Pt(111)电极与施加电势和水含量不超过50 wt%的函数。接触模式原子力显微镜(AFM)的力-距离曲线显示,对于所有三个DES,添加水可使界面纳米结构增加至约40 wt%,然后降低。这与离子液体完全不同,在离子液体中添加少量水会迅速降低界面纳米结构。对于纯DES,在OCP处在0.5 nm处仅测量到一个界面层,在40或50 wt%的水处,该界面层增加至从表面延伸约5 nm的3至6层。施加±0的电势。对于纯DES,向Pt电极施加25 V电压会使近表面层的数量增加至3。但是,当存在水时,施加的电势会削弱力曲线中的阶跃,并由短程指数衰减代替。对于含30 wt%或50 wt%的水的ChCl:EG,这种变化最为明显,因此使用循环伏安法对该系统进行了探测,这证实了界面纳米结构类似于盐溶液。



























 京公网安备 11010802027423号
京公网安备 11010802027423号