当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rapid Dissolution of Cinnabar in Crude Oils at Reservoir Temperatures Facilitated by Reduced Sulfur Ligands
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-08-28 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00096 Lars Lambertsson 1 , Charles J. Lord 2 , Wolfgang Frech 1 , Erik Björn 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-08-28 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00096 Lars Lambertsson 1 , Charles J. Lord 2 , Wolfgang Frech 1 , Erik Björn 1
Affiliation
|
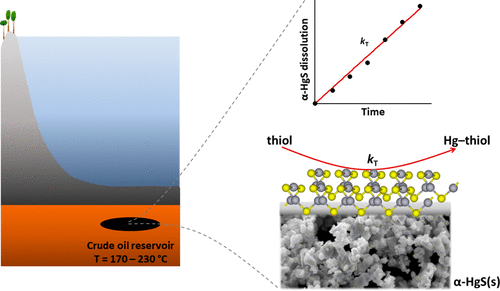
Mercury (Hg) is present in petrochemical samples, including crude oils, and the processing and use of petroleum products contribute to global Hg emissions. We present a refined theory on geochemical processes controlling Hg concentrations in crude oil by studying dissolution kinetics and solubility thermodynamics of cinnabar (α-HgS(s)) in different crude oils held at reservoir temperatures. In a black light crude oil, α-HgS(s) dissolved in an apparent zero-order reaction with a rate of 0.14–0.58 μmoles m–2 s–1 at 170–230 °C and an estimated activation energy of 43 kJ mol–1. For crude oil samples with a total sulfur concentration spanning 0.15–2.38% (w/w), the measured dissolution rate varied between 0.05 and 0.24 μmoles m–2 s–1 at 200 °C. Separate tests showed that thiols and, to a lesser extent, organic sulfides increased the solubility of α-HgS(s) in isooctane at room temperature compared to thiophenes, disulfides, and elemental sulfur. Long-term (14 days) α-HgS(s) solubility tests in a crude oil at 200 °C generated dissolved Hg concentrations in the 0.3% (w/w) range. The high α-HgS(s) dissolving capacity of the crude oils was more than 2 orders of magnitude greater than the highest reported Hg concentration in crude oils globally. On the basis of the kinetic and solubility data, it was further concluded that α-HgS(s) is not stable under typical petroleum reservoir conditions and would decompose to elemental mercury (Hg0). Our results suggest that source/reservoir temperature, abundance of reduced sulfur compounds in the crude oil, and dissolved Hg0 evasion processes are principal factors controlling the ultimate Hg concentration in a specific crude oil deposit.
中文翻译:

减少硫配体促进储层温度下朱砂在原油中的快速溶解
包括原油在内的石化样品中均存在汞(Hg),石油产品的加工和使用会导致全球汞排放。通过研究朱砂(α-HgS(s))在不同储层温度下在不同原油中的溶解动力学和溶解热力学,我们提出了控制原油中Hg浓度的地球化学过程的精细理论。在黑光原油中,α-HgS溶解于表观的零级反应中,在170-230°C时的速率为0.14-0.58μmolesm -2 s -1,估计活化能为43 kJ mol –1。对于总硫浓度为0.15–2.38%(w / w)的原油样品,测得的溶出速率在0.05至0.24μmolesm –2 s之间变化。200°C时为–1。单独的测试表明,与噻吩,二硫化物和元素硫相比,室温下硫醇和有机硫化物在较小程度上增加了α-HgS在异辛烷中的溶解度。在原油中于200°C进行的长期(14天)α-HgS溶解度测试得出的溶解Hg浓度在0.3%(w / w)范围内。原油的高α-HgS溶解能力比全球原油中报告的最高Hg浓度高2个数量级以上。根据动力学和溶解度数据,进一步得出结论,α-HgS在典型的石油储层条件下不稳定,会分解为元素汞(Hg 0)。我们的结果表明,源/储层温度,原油中还原的硫化合物的丰度以及溶解的Hg 0逃逸过程是控制特定原油矿床中最终Hg浓度的主要因素。
更新日期:2018-08-28
中文翻译:

减少硫配体促进储层温度下朱砂在原油中的快速溶解
包括原油在内的石化样品中均存在汞(Hg),石油产品的加工和使用会导致全球汞排放。通过研究朱砂(α-HgS(s))在不同储层温度下在不同原油中的溶解动力学和溶解热力学,我们提出了控制原油中Hg浓度的地球化学过程的精细理论。在黑光原油中,α-HgS溶解于表观的零级反应中,在170-230°C时的速率为0.14-0.58μmolesm -2 s -1,估计活化能为43 kJ mol –1。对于总硫浓度为0.15–2.38%(w / w)的原油样品,测得的溶出速率在0.05至0.24μmolesm –2 s之间变化。200°C时为–1。单独的测试表明,与噻吩,二硫化物和元素硫相比,室温下硫醇和有机硫化物在较小程度上增加了α-HgS在异辛烷中的溶解度。在原油中于200°C进行的长期(14天)α-HgS溶解度测试得出的溶解Hg浓度在0.3%(w / w)范围内。原油的高α-HgS溶解能力比全球原油中报告的最高Hg浓度高2个数量级以上。根据动力学和溶解度数据,进一步得出结论,α-HgS在典型的石油储层条件下不稳定,会分解为元素汞(Hg 0)。我们的结果表明,源/储层温度,原油中还原的硫化合物的丰度以及溶解的Hg 0逃逸过程是控制特定原油矿床中最终Hg浓度的主要因素。



























 京公网安备 11010802027423号
京公网安备 11010802027423号