当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unbiased Mass Spectrometry Elucidation of the Targets and Mechanisms of Activity-Based Probes: A Case Study Involving Sulfonyl Fluorides
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-09-07 00:00:00 , DOI: 10.1021/acschembio.8b00530 Thomas E. J. Chavas 1 , Matthew J. Fuchter 1 , Peter A. DiMaggio 2
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-09-07 00:00:00 , DOI: 10.1021/acschembio.8b00530 Thomas E. J. Chavas 1 , Matthew J. Fuchter 1 , Peter A. DiMaggio 2
Affiliation
|
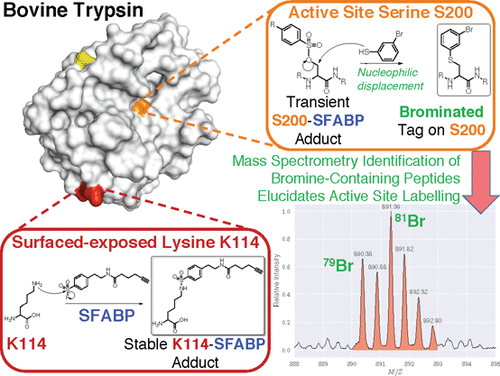
The elucidation of protein/drug interactions remains a major challenge in drug discovery. Liquid chromatography–tandem mass spectrometry has emerged as a tremendously powerful technology for this endeavor, but its full potential has yet to be realized owing in part to unresolved challenges in data analysis. Herein, we demonstrate how tandem mass spectrometry can comprehensively map small molecule/peptide adducts when combined with unconstrained sequencing. Using a published sulfonyl fluoride activity-based probe as a model system, this method enabled the discovery of several unreported sites of interaction with its target proteins. Crucially, this probe was found to undergo quantitative displacement and hydrolysis from the target protein’s active site. Isotopic labeling experiments provided a mechanistic rationale for the observed hydrolysis that involves neighboring-group participation. A chemical biology tagging strategy that leverages the probe’s observed lability was developed and shown to be compatible with the original small molecule inhibitor in discovery profiling experiments.
中文翻译:

基于活性的探针的目标和机理的无偏质谱阐明:涉及磺酰氟的案例研究
阐明蛋白质/药物相互作用仍然是药物开发中的主要挑战。液相色谱-串联质谱法已经成为一项非常强大的技术,但是其全部潜力尚未实现,这在一定程度上是由于数据分析中尚未解决的挑战。在本文中,我们证明了串联质谱法在与无约束测序结合时如何能够全面地绘制小分子/肽加合物。使用已发布的基于磺酰氟活性的探针作为模型系统,该方法能够发现几个未报告的与其靶蛋白相互作用的位点。至关重要的是,发现该探针从目标蛋白的活性位点发生定量置换和水解。同位素标记实验为观察到的涉及邻近基团参与的水解提供了机械原理。开发了一种利用探针观察到的不稳定性的化学生物学标记策略,该策略在发现分析实验中显示出与原始小分子抑制剂的相容性。
更新日期:2018-09-07
中文翻译:

基于活性的探针的目标和机理的无偏质谱阐明:涉及磺酰氟的案例研究
阐明蛋白质/药物相互作用仍然是药物开发中的主要挑战。液相色谱-串联质谱法已经成为一项非常强大的技术,但是其全部潜力尚未实现,这在一定程度上是由于数据分析中尚未解决的挑战。在本文中,我们证明了串联质谱法在与无约束测序结合时如何能够全面地绘制小分子/肽加合物。使用已发布的基于磺酰氟活性的探针作为模型系统,该方法能够发现几个未报告的与其靶蛋白相互作用的位点。至关重要的是,发现该探针从目标蛋白的活性位点发生定量置换和水解。同位素标记实验为观察到的涉及邻近基团参与的水解提供了机械原理。开发了一种利用探针观察到的不稳定性的化学生物学标记策略,该策略在发现分析实验中显示出与原始小分子抑制剂的相容性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号