当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Macroscopic and microscopic investigation of uranium elimination by Ca–Mg–Al-layered double hydroxide supported nanoscale zero valent iron†
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2018-09-07 00:00:00 , DOI: 10.1039/c8qi00779a Hongwei Pang 1, 2, 3, 4 , Yihan Wu 1, 2, 3, 4 , Shuyi Huang 1, 2, 3, 4 , Congcong Ding 5, 6, 7, 8 , Shun Li 1, 2, 3, 4 , Xiangxue Wang 1, 2, 3, 4, 9 , Shujun Yu 1, 2, 3, 4 , Zhongshan Chen 1, 2, 3, 4 , Gang Song 10, 11, 12 , Xiangke Wang 1, 2, 3, 4
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2018-09-07 00:00:00 , DOI: 10.1039/c8qi00779a Hongwei Pang 1, 2, 3, 4 , Yihan Wu 1, 2, 3, 4 , Shuyi Huang 1, 2, 3, 4 , Congcong Ding 5, 6, 7, 8 , Shun Li 1, 2, 3, 4 , Xiangxue Wang 1, 2, 3, 4, 9 , Shujun Yu 1, 2, 3, 4 , Zhongshan Chen 1, 2, 3, 4 , Gang Song 10, 11, 12 , Xiangke Wang 1, 2, 3, 4
Affiliation
|
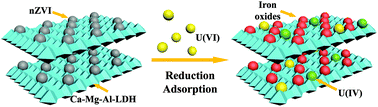
In the past few decades, uranium (235, 238U(VI)) has turned into a forefront environmental issue due to its extensive use in the nuclear industry and its toxicity and radioactivity. Herein, nanoscale zero valent iron (nZVI), which was supported on Ca–Mg–Al-layered double hydroxide (Ca–Mg–Al-LDH/nZVI), was fabricated via an in situ growth route and employed for U(VI) decontamination from aqueous solutions. Spectroscopy and microscopy (SEM, TEM, FTIR, XRD, BET, and XPS) technologies were applied for the investigation of the properties of Ca–Mg–Al-LDH/nZVI and the U(VI) elimination mechanism. Ca–Mg–Al-LDH/nZVI had a remarkable BET surface (426.8 m2 g−1), abundant functional groups (i.e., Fe–O, Al–O, –OH, etc.), high efficiency (4 h to achieve equilibrium) and large adsorption capacities (Qmax = 216.1 mg g−1) for U(VI) removal. The decontamination process of U(VI) was observed to be pH-dependent and ionic strength-independent, suggesting that the adsorption was predominated by inner-sphere surface coordination. XPS spectroscopy analyses indicated that the reduction and adsorption of nZVI and the adsorption of Ca–Mg–Al-LDH dominated U(VI) elimination on Ca–Mg–Al-LDH/nZVI. The environmentally friendly synthesis method, excellent physicochemical properties and remarkable removal performance suggested that Ca–Mg–Al-LDH/nZVI was applicable as a potential adsorbent for U(VI) decontamination from wastewater in environmental pollution remediation.
中文翻译:

Ca-Mg-Al层状双氢氧化物负载的纳米级零价铁对铀的宏观和微观研究†
在过去的几十年中,由于铀(235,238 U(VI))在核工业中的广泛使用及其毒性和放射性,已成为最重要的环境问题。本文中,通过原位生长途径制备了纳米零价铁(nZVI),该零价铁负载在Ca–Mg–Al层状双氢氧化物(Ca–Mg–Al-LDH / nZVI)上,并用于U(VI)从水溶液中去污。光谱和显微镜(SEM,TEM,FTIR,XRD,BET和XPS)技术用于研究Ca–Mg–Al-LDH / nZVI的性质以及U(VI)消除机理。Ca–Mg–Al-LDH / nZVI具有显着的BET表面(426.8 m 2 g-1),丰富的官能团(即Fe-O,Al-O,-OH等),高效率(达到平衡需要4小时)和对U的大吸附容量( Q max = 216.1 mg g -1) (六)拆除。观察到U( VI)的去污过程是pH依赖性和离子强度无关的,这表明吸附主要由内球表面配位作用决定。XPS光谱分析表明,nZVI的还原和吸附以及Ca-Mg-Al-LDH占主导的U( VI)在Ca–Mg–Al-LDH / nZVI上消除。环保的合成方法,出色的理化性质和出色的去除性能表明,Ca–Mg–Al-LDH / nZVI可作为环境污染修复中潜在的U(VI)废水去污吸附剂。
更新日期:2018-09-07
中文翻译:

Ca-Mg-Al层状双氢氧化物负载的纳米级零价铁对铀的宏观和微观研究†
在过去的几十年中,由于铀(235,238 U(VI))在核工业中的广泛使用及其毒性和放射性,已成为最重要的环境问题。本文中,通过原位生长途径制备了纳米零价铁(nZVI),该零价铁负载在Ca–Mg–Al层状双氢氧化物(Ca–Mg–Al-LDH / nZVI)上,并用于U(VI)从水溶液中去污。光谱和显微镜(SEM,TEM,FTIR,XRD,BET和XPS)技术用于研究Ca–Mg–Al-LDH / nZVI的性质以及U(VI)消除机理。Ca–Mg–Al-LDH / nZVI具有显着的BET表面(426.8 m 2 g-1),丰富的官能团(即Fe-O,Al-O,-OH等),高效率(达到平衡需要4小时)和对U的大吸附容量( Q max = 216.1 mg g -1) (六)拆除。观察到U( VI)的去污过程是pH依赖性和离子强度无关的,这表明吸附主要由内球表面配位作用决定。XPS光谱分析表明,nZVI的还原和吸附以及Ca-Mg-Al-LDH占主导的U( VI)在Ca–Mg–Al-LDH / nZVI上消除。环保的合成方法,出色的理化性质和出色的去除性能表明,Ca–Mg–Al-LDH / nZVI可作为环境污染修复中潜在的U(VI)废水去污吸附剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号