当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rhodium(iii)-catalyzed C–H activation at the C4-position of indole: switchable hydroarylation and oxidative Heck-type reactions of maleimides†
Chemical Communications ( IF 4.9 ) Pub Date : 2018-09-06 00:00:00 , DOI: 10.1039/c8cc06264a Mahadev Sharanappa Sherikar 1, 2, 3, 4 , Raja Kapanaiah 1, 2, 3, 4 , Veeranjaneyulu Lanke 1, 2, 3, 4 , Kandikere Ramaiah Prabhu 1, 2, 3, 4
Chemical Communications ( IF 4.9 ) Pub Date : 2018-09-06 00:00:00 , DOI: 10.1039/c8cc06264a Mahadev Sharanappa Sherikar 1, 2, 3, 4 , Raja Kapanaiah 1, 2, 3, 4 , Veeranjaneyulu Lanke 1, 2, 3, 4 , Kandikere Ramaiah Prabhu 1, 2, 3, 4
Affiliation
|
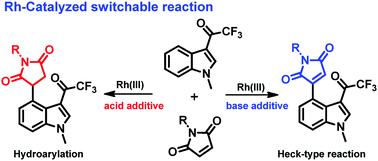
A Rh(III)-catalyzed C–H activation of indole at the C4-position leading to novel and switchable functionalization has been reported by employing a weakly co-ordinating COCF3 group as a directing group. An additive plays an important role in switching the selectivity between 1,4-addition products and Heck-type products. An acid additive led to the formation of 1,4-addition products whereas a base additive promotes the formation of Heck-type products. Deuteration studies and control experiments were helpful to propose the mechanism.
中文翻译:

铑(iii)催化的吲哚C4位上的C–H活化:马来酰亚胺的可切换的氢芳基化和氧化性Heck型反应†
通过弱配位的COCF 3基团作为指导基团,已报道了Rh(III)催化的C4-位吲哚在CH4上的CH活化,从而导致新颖且可转换的官能化。添加剂在1,4-加成产物和Heck型产物之间切换选择性方面起着重要作用。酸添加剂导致形成1,4-加成产物,而碱添加剂则促进形成Heck型产物。氘代研究和控制实验有助于提出机理。
更新日期:2018-09-06
中文翻译:

铑(iii)催化的吲哚C4位上的C–H活化:马来酰亚胺的可切换的氢芳基化和氧化性Heck型反应†
通过弱配位的COCF 3基团作为指导基团,已报道了Rh(III)催化的C4-位吲哚在CH4上的CH活化,从而导致新颖且可转换的官能化。添加剂在1,4-加成产物和Heck型产物之间切换选择性方面起着重要作用。酸添加剂导致形成1,4-加成产物,而碱添加剂则促进形成Heck型产物。氘代研究和控制实验有助于提出机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号