Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2018-09-06 , DOI: 10.1016/j.bmcl.2018.09.008 Wenlong Kong , Yinhe Bao , Qianjun Ma , Hui Xu
|
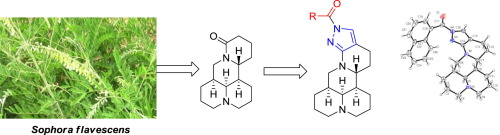
In continuation of our program aimed at the development of new natural product-based pesticides, a series of novel pyrazolomatrine derivatives were prepared by structural modifications of matrine, isolated as a quinolizidine alkaloid from the roots of Sophora flave. Their structures were confirmed by 1H NMR, HRMS, etc. Moreover, the steric structures of three compounds were determined by single-crystal X-ray diffraction. Among all derivatives, 19-(naphthyl-2-oyl)pyrazolomatrine (5y) showed 3.13-fold more potent acaricidal activity than its precusor matrine against Tetranychus cinnabarinus; 19-(4-methylbenzoyl)pyrazolomatrine (5j) and 19-(3,5-dimethylbenzoyl)pyrazolomatrine (5k) displayed the promising aphicidal activity against Aphis citricola van der. Their structure-activity relationships were also observed.
中文翻译:

新型吡唑并苦参碱衍生物的合成及生物活性
在我们旨在开发新的基于天然产物的农药的计划的延续中,通过苦参碱的结构修饰制备了一系列新型的吡唑并苦参碱衍生物,从苦参的根中分离出喹诺唑烷生物碱的形式。它们的结构通过1 H NMR,HRMS等证实。此外,三种化合物的空间结构通过单晶X射线衍射确定。在所有衍生物中,19-(萘-2-甲酰基)吡唑并苦参碱(5y)的抗杀螨活性比其先驱苦参碱对朱砂叶螨的杀螨活性高3.13倍;19-(4-甲基苯甲酰基)吡唑并苦参碱(5j)和19-(3,5-二甲基苯甲酰基)吡唑并苦参碱(5k)表现出对抗柠檬蚜的有希望的杀蚜活性。还观察到它们的结构-活性关系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号