当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic and Kinetic Investigations on the Thermal Unimolecular Reaction of Heptafluoroisobutyronitrile
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-09-06 00:00:00 , DOI: 10.1021/acs.jpca.8b07189 Xiaojuan Yu 1 , Hua Hou 1 , Baoshan Wang 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-09-06 00:00:00 , DOI: 10.1021/acs.jpca.8b07189 Xiaojuan Yu 1 , Hua Hou 1 , Baoshan Wang 1
Affiliation
|
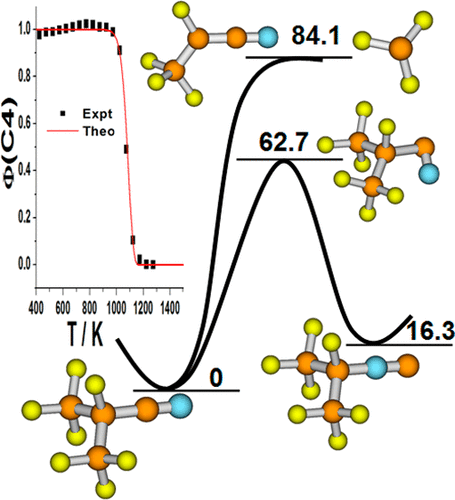
Heptafluoroisobutyronitrile (C4) has been utilized as a dielectric compound to replace sulfur hexafluoride for the sake of environmental concern. Energetic profiles of the potential energy surface for the unimolecular reaction of C4 were calculated using density functional theory (M06-2X), quadratic complete basis set (CBS-QB3), Gaussian-4, multireference Rayleigh–Schrodinger perturbation theory (RS2, RS2C), and state-averaged multiconfiguration self-consistent-field (SA-MCSCF) ab initio methods. Among 38 production channels, the most energetically accessible reaction path is the three-centered rearrangement of cyanide to isocyanide. The C—CF3 bond appears to be the weakest bond in C4(X1A′) and the symmetry-breaking C—CF3 bond cleavage undergoes to form the ground-state CF3(X2A1) and CF3CFCN (X2A″) radicals. All the possible isomerization pathways involving F- and CF3-migration together with the concerted elimination and stepwise decomposition routes were revealed for C4 and i-C4. Various isomers and potential toxic byproducts including FCN, CF3CN, C2F5CN, CF2═CFCF3, CF2═CFCN, CF4, C2F6, and alkyne compounds have been identified for the first time. Master equation analysis has been carried out to obtain the temperature- and pressure-dependent thermal rate constants. Theoretical kinetics for the loss of C4 due to pyrolysis is in good agreement with the temperature-ramped flow-tube experiment. The present theoretical work provides new insights on the thermal stability and chemical reactivity of C4. Moreover, i-C4 and C2F6 are proposed to be the potential characteristic gas molecules to monitor the insulation breakdown of C4 in the electric equipment.
中文翻译:

七氟异丁腈热单分子反应的机理和动力学研究
出于对环境的关注,七氟异丁腈(C4)已被用作介电化合物来代替六氟化硫。使用密度泛函理论(M06-2X),二次完全基集(CBS-QB3),高斯4,多参考瑞利-薛定inger摄动理论(RS2,RS2C)计算C4单分子反应的势能面的能谱,以及状态平均的多配置自洽字段(SA-MCSCF)从头计算方法。在38个生产通道中,最容易获得能量的反应路径是氰化物向异氰化物的三中心重排。C-CF 3键似乎是C4(X 1 A')和破坏对称的C-CF 3中最弱的键键断裂经历形成基态CF 3(X 2 A 1)和CF 3 CFCN(X 2 A'')自由基。揭示了C4和i-C4涉及F和CF 3迁移的所有可能的异构化途径,以及一致的消除和逐步分解途径。各种异构体和潜在的毒性副产物,包括FCN,CF 3 CN,C 2 ˚F 5 CN,CF 2 = CFCF 3,CF 2 ═CFCN,CF 4,C 2 ˚F 6和炔烃化合物已被首次鉴定。已经进行了主方程分析,以获得温度和压力相关的热速率常数。由于热解而损失的C4的理论动力学与温度梯度流管实验非常吻合。目前的理论工作为C4的热稳定性和化学反应性提供了新的见解。此外,提出了i-C4和C 2 F 6作为监测电气设备中C4绝缘击穿的潜在特征气体分子。
更新日期:2018-09-06
中文翻译:

七氟异丁腈热单分子反应的机理和动力学研究
出于对环境的关注,七氟异丁腈(C4)已被用作介电化合物来代替六氟化硫。使用密度泛函理论(M06-2X),二次完全基集(CBS-QB3),高斯4,多参考瑞利-薛定inger摄动理论(RS2,RS2C)计算C4单分子反应的势能面的能谱,以及状态平均的多配置自洽字段(SA-MCSCF)从头计算方法。在38个生产通道中,最容易获得能量的反应路径是氰化物向异氰化物的三中心重排。C-CF 3键似乎是C4(X 1 A')和破坏对称的C-CF 3中最弱的键键断裂经历形成基态CF 3(X 2 A 1)和CF 3 CFCN(X 2 A'')自由基。揭示了C4和i-C4涉及F和CF 3迁移的所有可能的异构化途径,以及一致的消除和逐步分解途径。各种异构体和潜在的毒性副产物,包括FCN,CF 3 CN,C 2 ˚F 5 CN,CF 2 = CFCF 3,CF 2 ═CFCN,CF 4,C 2 ˚F 6和炔烃化合物已被首次鉴定。已经进行了主方程分析,以获得温度和压力相关的热速率常数。由于热解而损失的C4的理论动力学与温度梯度流管实验非常吻合。目前的理论工作为C4的热稳定性和化学反应性提供了新的见解。此外,提出了i-C4和C 2 F 6作为监测电气设备中C4绝缘击穿的潜在特征气体分子。


























 京公网安备 11010802027423号
京公网安备 11010802027423号