Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-09-06 , DOI: 10.1016/j.bioorg.2018.08.031 Noha G. Mohamed , Mahmoud M. Sheha , Hoda Y. Hassan , Lina Jamil M. Abdel-Hafez , Farghaly A. Omar
|
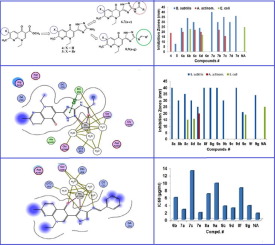
Four series of triazolylnaphthyridinone derivatives were synthesized as structural surrogates of nalidixic acid. The targeted derivatives involve: 3-(5-acylamino-2H-1,2,4-triazol-3-yl)-naphtyridin-4-ones 6(a-e); 3-(5-benzylidineamino-2H-1,2,4-triazol-3-yl)-naphthyridin-4-ones 8(a-g) and their 6-bromonaphthyridin-4-one analogs 7(a-e); 9(a-g). The synthesized compounds were evaluated In vitro for their antimicrobial activity against selected resistant strains of G+ve, G−ve, and Mycobacterium phlei. The results revealed remarkable selectivity, of the tested compounds, against Bacillus subtilis and Aggregatibacter actinomycetemcomitans, which are resistant to nalidixic acid. The growth inhibition zones were ranging from 20 to 40 mm at 10 mg/ml and the respective MIC-values ∼3.68–6.3 µM. The results illustrate that the 6-bromo derivatives 7(a-e) and 9(a-g) were more potent than the non-brominated counterparts 6(a-e) and 8(a-e) respectively. Inhibition of E. coli DNA-gyrase supercoiling activity is also evaluated. The 5-(4-methoxybanzamido)-triazolyl-6-bromonaphthyridinone (7e) exhibits IC50 = 1.94 μg/ml, which is comparable to that of nalidixic acid (IC50: 1.74 μg/ml). In addition, the most prominent IC50-values are displayed by: (7a; IC50: 2.77 μg/ml); (8g; IC50: 3.78 μg/ml); and (9d; IC50: 3.21 μg/ml). Molecular docking to the active site of DNA–gyrase cleavage complex of Acinetobacter baumannii (PDB code: 2xkk) co-crystallized with moxifloxacin revealed similar binding modes in addition to new interactions. Assessment of drug-likeness characteristics illustrate that the synthesized compounds showed agreement to Lipinski's and Veper's parameters. The study could offer an exceptional framework that may lead to the discovery of new potent antimicrobial agents.
中文翻译:

3-(5-氨基-(2H)-1,2,4-三唑--3-基]-萘啶酮类化合物的合成,抗菌活性和分子建模研究
合成了四个系列的三唑基萘并吡啶酮衍生物作为萘啶酸的结构替代物。目标衍生物包括:3-(5-酰基氨基-2H-1,2,4-三唑-3-基)-萘啶-4-酮6(ae) ; 3-(5-亚苄基氨基-2H-1,2,4-三唑-3-基)-萘啶-4-酮8(ag)及其6-溴萘吡啶-4-酮类似物7(ae) ; 9(ag)。在体外评估了合成的化合物对选定的G + ve,G-ve和分枝杆菌耐药菌株的抗菌活性。结果表明,测试化合物对枯草芽孢杆菌和对萘啶酸具有抗性的放线杆菌。10 mg / ml时,生长抑制区的范围为20至40 mm,相应的MIC-值约为3.68–6.3 µM。结果表明6-溴衍生物7(ae)和9(ag)分别比非溴代对应物6(ae)和8(ae)更有效。还评估了对大肠杆菌DNA旋转酶超螺旋活性的抑制作用。的5-(4- methoxybanzamido) -三唑基-6- bromonaphthyridinone (7E)表现出IC 50 = 1.94微克/毫升,这相当于该萘啶酸的(IC50:1.74μg/ ml)。另外,最突出的IC 50值显示为:( 7a; IC 50:2.77μg/ ml);( 8g; IC 50:3.78μg/ ml);和(9D; IC 50: 3.21微克/毫升)。分子对接鲍曼不动杆菌DNA-旋切酶裂解复合物的活性位点与莫西沙星共结晶的(PDB代码:2xkk)除了新的相互作用外,还显示出相似的结合模式。药物相似性特征的评估表明,合成的化合物显示出与Lipinski's和Veper's参数一致。该研究可能会提供一个例外的框架,从而可能导致发现新的有效抗菌剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号