当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Can molten carbonate be a non-metal catalyst for CO oxidation?
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-09-05 , DOI: 10.1039/c8nj02462f Jingjing Tong 1, 2, 3, 4, 5 , Xueling Lei 1, 2, 3, 4, 5 , Peng Zhang 4, 5, 6, 7 , Kevin Huang 4, 5, 6, 7 , Godwin Mbamalu 1, 2, 3, 4, 5 , Changyong Qin 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-09-05 , DOI: 10.1039/c8nj02462f Jingjing Tong 1, 2, 3, 4, 5 , Xueling Lei 1, 2, 3, 4, 5 , Peng Zhang 4, 5, 6, 7 , Kevin Huang 4, 5, 6, 7 , Godwin Mbamalu 1, 2, 3, 4, 5 , Changyong Qin 1, 2, 3, 4, 5
Affiliation
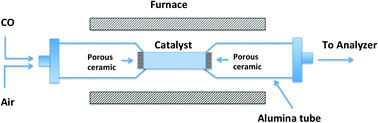
|
For the first time, we have examined molten carbonate as a non-metal catalyst for CO oxidation in the temperature range of 300–600 °C. The reaction mechanism was analyzed using a classic Langmuir–Hinshelwood model combined with DFT calculations. It was found that the conversion of CO is greatly enhanced by molten carbonate at about 400 °C and increased to 96% at 500 °C. The reaction process involves four steps, including (1) dissociative adsorption of oxygen, (2) adsorption of CO, (3) surface reaction, and (4) desorption of CO2. DFT modeling reveals the formation of (C2O4)2− and (CO4)2− as the intermediate species, and that the first two steps are exothermic and preferred by chemical equilibrium. The energy barrier of oxygen dissociation to form CO42− is calculated to be 23.0 kcal mol−1, which is in good agreement with the measured overall activation energy of 19.1 kcal mol−1. However, the surface reaction (step 3) has a low energy barrier of 10.8 kcal mol−1 only. This confirms that the oxygen dissociation is the rate determing step in the whole process. Further analysis of the reaction kinetics indicates that the reaction is affected by the CO concentration. With low CO concentration, the reaction is first order with respect to CO and half order to O2. From the current report, it has been proven that molten carbonate can serve as an efficient catalyst for CO oxidation and potentially for other oxidation reactions in the temperature range of 400–600 °C. More studies are demanded to further investigate the reaction mechanism and explore more potential industrial applications.
中文翻译:

熔融碳酸盐可以作为CO氧化的非金属催化剂吗?
我们首次研究了熔融碳酸盐作为300-600°C温度范围内CO氧化的非金属催化剂。使用经典的Langmuir-Hinshelwood模型结合DFT计算分析了反应机理。发现在约400℃下熔融的碳酸盐大大提高了CO的转化率,并在500℃下提高了96%。反应过程包括四个步骤,包括(1)氧的解离吸附,(2)CO的吸附,(3)表面反应和(4)CO 2的解吸。DFT建模揭示了(C 2 O 4)2−和(CO 4)2−的形成作为中间物种,并且前两个步骤是放热的,并且通过化学平衡是优选的。氧离解形成CO 4 2-的能垒经计算为23.0 kcal mol -1,与测得的19.1 kcal mol -1的总活化能非常吻合。然而,表面反应(步骤3)仅具有10.8kcal mol -1的低能垒。这证实了氧离解是整个过程中速率决定的步骤。反应动力学的进一步分析表明反应受到CO浓度的影响。在低CO浓度下,反应相对于CO是一阶,对于O 2是半阶。根据当前报告,已证明熔融碳酸盐可作为有效的催化剂用于CO氧化以及潜在地在400-600°C的温度范围内进行其他氧化反应。需要更多的研究来进一步研究反应机理并探索更多潜在的工业应用。
更新日期:2018-09-25
中文翻译:

熔融碳酸盐可以作为CO氧化的非金属催化剂吗?
我们首次研究了熔融碳酸盐作为300-600°C温度范围内CO氧化的非金属催化剂。使用经典的Langmuir-Hinshelwood模型结合DFT计算分析了反应机理。发现在约400℃下熔融的碳酸盐大大提高了CO的转化率,并在500℃下提高了96%。反应过程包括四个步骤,包括(1)氧的解离吸附,(2)CO的吸附,(3)表面反应和(4)CO 2的解吸。DFT建模揭示了(C 2 O 4)2−和(CO 4)2−的形成作为中间物种,并且前两个步骤是放热的,并且通过化学平衡是优选的。氧离解形成CO 4 2-的能垒经计算为23.0 kcal mol -1,与测得的19.1 kcal mol -1的总活化能非常吻合。然而,表面反应(步骤3)仅具有10.8kcal mol -1的低能垒。这证实了氧离解是整个过程中速率决定的步骤。反应动力学的进一步分析表明反应受到CO浓度的影响。在低CO浓度下,反应相对于CO是一阶,对于O 2是半阶。根据当前报告,已证明熔融碳酸盐可作为有效的催化剂用于CO氧化以及潜在地在400-600°C的温度范围内进行其他氧化反应。需要更多的研究来进一步研究反应机理并探索更多潜在的工业应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号