当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Biological Evaluation of Fentanyl Analogues Modified at Phenyl Groups with Alkyls.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-09-14 , DOI: 10.1021/acschemneuro.8b00363 Yajuan Qin 1 , Luofan Ni 1 , Jiawei Shi 1 , Zhiying Zhu 1 , Saijian Shi 1 , Ai-Leen Lam 2 , Julia Magiera 2 , Sunderajhan Sekar 2 , Andy Kuo 2 , Maree T Smith 2 , Tingyou Li 1, 3
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-09-14 , DOI: 10.1021/acschemneuro.8b00363 Yajuan Qin 1 , Luofan Ni 1 , Jiawei Shi 1 , Zhiying Zhu 1 , Saijian Shi 1 , Ai-Leen Lam 2 , Julia Magiera 2 , Sunderajhan Sekar 2 , Andy Kuo 2 , Maree T Smith 2 , Tingyou Li 1, 3
Affiliation
|
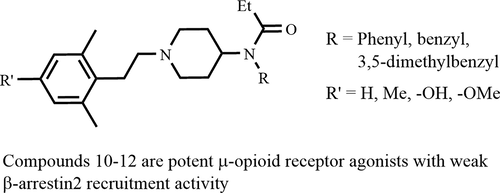
A series of fentanyl analogues modified at the phenyl group of the phenethyl with alkyl and/or hydroxyl and alkoxy, and the phenyl group in the anilido moiety replaced with benzyl or substituted benzyl, were synthesized. The in vitro opioid receptor functional activity of these compounds was evaluated by assessment of their ability to modulate forskolin-stimulated cAMP accumulation and by their ability to induce β-arrestin2 recruitment. Compound 12 is a potent μ-opioid (MOP) receptor agonist, a potent κ-opioid (KOP) receptor antagonist with weak β-arrestin2 recruitment activity. Compounds 10 and 11 are potent MOP receptor agonists with weak δ-opioid (DOP) receptor antagonist activity and moderate KOP receptor antagonist activity as well as weak β-arrestin2 recruitment activity at the MOP receptor. These compounds are promising leads for discovery of potent opioid analgesics with reduced side effects relative to clinically available strong opioid analgesics.
中文翻译:

苯烷基修饰的芬太尼类似物的合成及生物评价。
合成了一系列在苯乙基的苯基上被烷基和/或羟基和烷氧基改性的芬太尼类似物,以及苯胺基部分中的苯基被苄基或取代的苄基所取代。通过评估它们调节毛喉素刺激的cAMP积累的能力以及诱导β-arrestin2募集的能力,评估了这些化合物的体外阿片受体功能活性。化合物12是有效的μ阿片类药物(MOP)受体激动剂,是一种具有弱β-arrestin2募集活性的有效κ-阿片类药物(KOP)受体拮抗剂。化合物10和11是有效的MOP受体激动剂,其具有弱的δ-阿片样物质(DOP)受体拮抗剂活性和中等的KOP受体拮抗剂活性以及在MOP受体处的β-arrestin2募集活性弱。
更新日期:2018-09-04
中文翻译:

苯烷基修饰的芬太尼类似物的合成及生物评价。
合成了一系列在苯乙基的苯基上被烷基和/或羟基和烷氧基改性的芬太尼类似物,以及苯胺基部分中的苯基被苄基或取代的苄基所取代。通过评估它们调节毛喉素刺激的cAMP积累的能力以及诱导β-arrestin2募集的能力,评估了这些化合物的体外阿片受体功能活性。化合物12是有效的μ阿片类药物(MOP)受体激动剂,是一种具有弱β-arrestin2募集活性的有效κ-阿片类药物(KOP)受体拮抗剂。化合物10和11是有效的MOP受体激动剂,其具有弱的δ-阿片样物质(DOP)受体拮抗剂活性和中等的KOP受体拮抗剂活性以及在MOP受体处的β-arrestin2募集活性弱。


























 京公网安备 11010802027423号
京公网安备 11010802027423号