当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium triggered diene formation from nitro allylic compounds: a versatile entry into naphthalene derivatives†
Chemical Communications ( IF 4.9 ) Pub Date : 2018-09-05 00:00:00 , DOI: 10.1039/c8cc06536e Mansour Dolè Kerim 1, 2, 3, 4, 5 , Shuanglong Jia 1, 2, 3, 4, 5 , Chrysoula Theodorakidou 1, 2, 3, 4, 5 , Sébastien Prévost 1, 2, 3, 4, 5 , Laurent El Kaïm 1, 2, 3, 4, 5
Chemical Communications ( IF 4.9 ) Pub Date : 2018-09-05 00:00:00 , DOI: 10.1039/c8cc06536e Mansour Dolè Kerim 1, 2, 3, 4, 5 , Shuanglong Jia 1, 2, 3, 4, 5 , Chrysoula Theodorakidou 1, 2, 3, 4, 5 , Sébastien Prévost 1, 2, 3, 4, 5 , Laurent El Kaïm 1, 2, 3, 4, 5
Affiliation
|
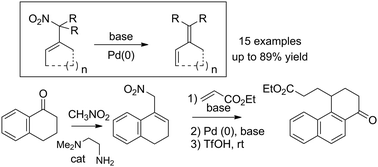
Nitro allylic derivatives were converted into dienes through the elimination of the nitro group under basic treatment, in the presence of a palladium catalyst. This reaction probably involves the formation of a palladium π-allyl complex followed by a base-promoted β-hydride elimination. This reaction, combined with the condensation of ketones with nitromethane and the functionalization of the resulting nitrocycloalkenes, constitutes a very powerful synthetic tool for the formation of dienes. Particular attention has been brought to the application of this methodology to the formation of 1-substituted naphthalenes from 1-tetralone.
中文翻译:

钯引发硝基烯丙基烯丙基化合物形成二烯:萘衍生物的广泛用途†
在钯催化剂的存在下,在碱性处理下,通过消除硝基,将硝基烯丙基衍生物转化为二烯。该反应可能涉及形成钯π-烯丙基络合物,然后消除碱促进的β-氢化物。该反应与酮与硝基甲烷的缩合以及所得硝基环烯烃的官能化相结合,构成了用于形成二烯的非常强大的合成工具。特别注意了该方法在从1-四氢萘酮形成1-取代萘中的应用。
更新日期:2018-09-05
中文翻译:

钯引发硝基烯丙基烯丙基化合物形成二烯:萘衍生物的广泛用途†
在钯催化剂的存在下,在碱性处理下,通过消除硝基,将硝基烯丙基衍生物转化为二烯。该反应可能涉及形成钯π-烯丙基络合物,然后消除碱促进的β-氢化物。该反应与酮与硝基甲烷的缩合以及所得硝基环烯烃的官能化相结合,构成了用于形成二烯的非常强大的合成工具。特别注意了该方法在从1-四氢萘酮形成1-取代萘中的应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号