当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Twist sense control in terminally functionalized ortho-phenylenes†
Chemical Science ( IF 8.4 ) Pub Date : 2018-09-05 00:00:00 , DOI: 10.1039/c8sc02821d Gopi Nath Vemuri 1, 2, 3, 4 , Rathiesh R. Pandian 1, 2, 3, 4 , Brian J. Spinello 1, 2, 3, 4 , Erika B. Stopler 1, 2, 3, 4 , Zacharias J. Kinney 1, 2, 3, 4 , C. Scott Hartley 1, 2, 3, 4
Chemical Science ( IF 8.4 ) Pub Date : 2018-09-05 00:00:00 , DOI: 10.1039/c8sc02821d Gopi Nath Vemuri 1, 2, 3, 4 , Rathiesh R. Pandian 1, 2, 3, 4 , Brian J. Spinello 1, 2, 3, 4 , Erika B. Stopler 1, 2, 3, 4 , Zacharias J. Kinney 1, 2, 3, 4 , C. Scott Hartley 1, 2, 3, 4
Affiliation
|
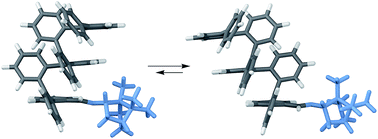
Many abiotic foldamers are based on achiral repeat units but adopt chiral geometries, especially helices. In these systems, there is no inherent preference for one handedness of the fold; however, it is well-established that the point chirality of substituents can be communicated to the helix. This capability represents a basic level of control over folding that is necessary for applications in molecular recognition and in the assembly of higher-order structures. The ortho-phenylenes are a structurally simple class of aromatic foldamers that fold into helices driven by arene–arene stacking interactions. Although their folding is now reasonably well-understood, access to o-phenylenes enriched in one twist sense has been limited to resolution, yielding conformationally dynamic samples that racemize over the course of minutes to hours. Here, we report a detailed structure–property study of chiral induction from o-phenylene termini using a combination of NMR spectroscopy, CD spectroscopy, and computational chemistry. We uncover mechanistic details of chiral induction and show that the same substituents can give effective twist sense control in opposite directions in mixtures of interconverting conformers; that is, they are “ambidextrous”. This behavior should be general and can be rationalized using a simple model based on sterics, noting that arene–arene stacking is, to a first approximation, unaffected by flipping either partner. We demonstrate control over this mechanism by showing that chiral groups can be chosen such that they both favor one orientation and provide effective chiral induction.
中文翻译:

末端官能化邻苯撑中的扭曲感控制†
许多非生物折叠剂均基于非手性重复单元,但采用手性几何结构,尤其是螺旋结构。在这些系统中,没有固有的偏向性。然而,已经公认可以将取代基的点手性传递给螺旋。这种功能代表了对折叠的基本控制,这对于分子识别和高阶结构组装中的应用是必不可少的。的邻-phenylenes是一种结构简单的类折叠成通过驱动螺旋芳香族foldamers的芳烃-芳烃堆积相互作用。虽然它们的折叠现在已经相当好理解,获得Ø在一种扭曲意义上富集的亚苯基苯仅限于拆分,可生成在数分钟至数小时内消旋的构象动力学样品。在这里,我们报告从手性诱导的详细结构和性能研究Ø-亚苯基末端使用NMR光谱,CD光谱和计算化学的组合。我们揭示了手性诱导的机理细节,并表明在相互转化的构象异构体的混合物中,相同的取代基可以在相反的方向上提供有效的扭曲感控制。也就是说,它们是“灵巧的”。这种行为应该是普遍的,并且可以使用基于空间的简单模型进行合理化,并指出,芳烃–芳烃的堆积在第一近似情况下不受翻转任何一个伙伴的影响。我们通过显示可以选择手性基团来证明对这一机制的控制,使得它们都有利于一个方向并提供有效的手性诱导。
更新日期:2018-09-05
中文翻译:

末端官能化邻苯撑中的扭曲感控制†
许多非生物折叠剂均基于非手性重复单元,但采用手性几何结构,尤其是螺旋结构。在这些系统中,没有固有的偏向性。然而,已经公认可以将取代基的点手性传递给螺旋。这种功能代表了对折叠的基本控制,这对于分子识别和高阶结构组装中的应用是必不可少的。的邻-phenylenes是一种结构简单的类折叠成通过驱动螺旋芳香族foldamers的芳烃-芳烃堆积相互作用。虽然它们的折叠现在已经相当好理解,获得Ø在一种扭曲意义上富集的亚苯基苯仅限于拆分,可生成在数分钟至数小时内消旋的构象动力学样品。在这里,我们报告从手性诱导的详细结构和性能研究Ø-亚苯基末端使用NMR光谱,CD光谱和计算化学的组合。我们揭示了手性诱导的机理细节,并表明在相互转化的构象异构体的混合物中,相同的取代基可以在相反的方向上提供有效的扭曲感控制。也就是说,它们是“灵巧的”。这种行为应该是普遍的,并且可以使用基于空间的简单模型进行合理化,并指出,芳烃–芳烃的堆积在第一近似情况下不受翻转任何一个伙伴的影响。我们通过显示可以选择手性基团来证明对这一机制的控制,使得它们都有利于一个方向并提供有效的手性诱导。


























 京公网安备 11010802027423号
京公网安备 11010802027423号