当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intramolecular Noncovalent Carbon Bonding Interaction Stabilizes the cis Conformation in Acylhydrazones.
ChemPlusChem ( IF 3.4 ) Pub Date : 2018-09-19 , DOI: 10.1002/cplu.201800329 Muhammad Moazzam Naseer 1 , Majid Hussain 1 , Antonio Bauzá 2 , Kong Mun Lo 3 , Antonio Frontera 2
ChemPlusChem ( IF 3.4 ) Pub Date : 2018-09-19 , DOI: 10.1002/cplu.201800329 Muhammad Moazzam Naseer 1 , Majid Hussain 1 , Antonio Bauzá 2 , Kong Mun Lo 3 , Antonio Frontera 2
Affiliation
|
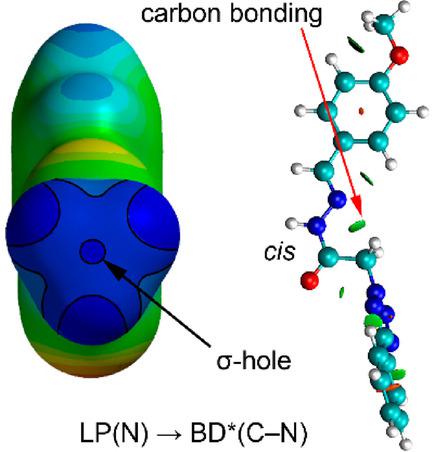
Noncovalent carbon bonding, a recently explored σ-hole interaction, was hitherto supposed to be a weak and structure-guided interaction. Here, its role in the intramolecular stabilization of the cis conformation of the amide moiety in acylhydrazones is described. The calculations reveal an electron donation from the lone pair of the nitrogen atom to the empty antibonding C-N orbital [LP(N)→BD*(C-N)] with a concomitant stabilization energy of E(2) =1.2 kcal mol-1 .
中文翻译:

分子内非共价碳键相互作用可稳定酰基hydr中的顺式构象。
迄今为止,非共价碳键(一种最近探索的σ-孔相互作用)被认为是一种弱的且受结构引导的相互作用。在此,描述了其在酰基hydr中酰胺部分的顺式构象的分子内稳定中的作用。计算结果表明,一个电子从氮原子的孤对向空的反键CN轨道[LP(N)→BD *(CN)]供电,伴随的稳定能量为E(2)= 1.2 kcal mol-1。
更新日期:2018-09-19
中文翻译:

分子内非共价碳键相互作用可稳定酰基hydr中的顺式构象。
迄今为止,非共价碳键(一种最近探索的σ-孔相互作用)被认为是一种弱的且受结构引导的相互作用。在此,描述了其在酰基hydr中酰胺部分的顺式构象的分子内稳定中的作用。计算结果表明,一个电子从氮原子的孤对向空的反键CN轨道[LP(N)→BD *(CN)]供电,伴随的稳定能量为E(2)= 1.2 kcal mol-1。



























 京公网安备 11010802027423号
京公网安备 11010802027423号