当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Peptide-Functionalized Hydrogel Cubes for Active Tumor Cell Targeting.
Biomacromolecules ( IF 6.2 ) Pub Date : 2018-09-12 , DOI: 10.1021/acs.biomac.8b01088 Bing Xue , Veronika Kozlovskaya , Mohammad Asif Sherwani , Sithira Ratnayaka , Shahriar Habib , Theron Anderson , Marina Manuvakhova 1 , Lidija Klampfer 1 , Nabiha Yusuf , Eugenia Kharlampieva
Biomacromolecules ( IF 6.2 ) Pub Date : 2018-09-12 , DOI: 10.1021/acs.biomac.8b01088 Bing Xue , Veronika Kozlovskaya , Mohammad Asif Sherwani , Sithira Ratnayaka , Shahriar Habib , Theron Anderson , Marina Manuvakhova 1 , Lidija Klampfer 1 , Nabiha Yusuf , Eugenia Kharlampieva
Affiliation
|
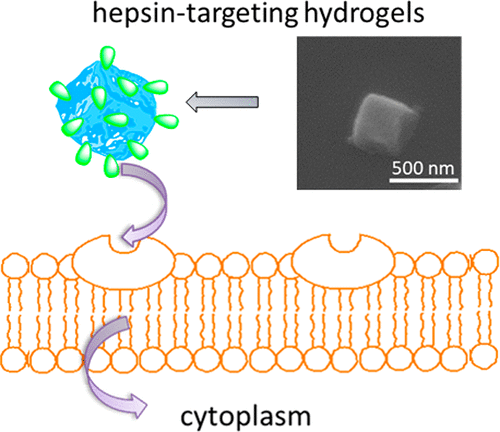
Conjugation of bioactive targeting molecules to nano- or micrometer-sized drug carriers is a pivotal strategy to improve their therapeutic efficiency. Herein, we developed pH- and redox-sensitive hydrogel particles with a surface-conjugated cancer cell targeting ligand for specific tumor-targeting and controlled release of the anticancer drug doxorubicin. The poly(methacrylic acid) (PMAA) hydrogel cubes of 700 nm and 2 μm with a hepsin-targeting (IPLVVPL) surface peptide are produced through multilayer polymer assembly on sacrificial cubical mesoporous cores. Direct peptide conjugation to the disulfide-stabilized hydrogels through a thiol-amine reaction does not compromise the structural integrity, hydrophilicity, stability in serum, or pH/redox sensitivity but does affect internalization by cancer cells. The cell uptake kinetics and the ultimate extent of internalization are controlled by the cell type and hydrogel size. The peptide modification significantly promotes the uptake of the 700 nm hydrogels by hepsin-positive MCF-7 cells due to ligand-receptor recognition but has a negligible effect on the uptake of 2 μm PMAA hydrogels. The selectivity of 700 nm IPLVVPL-PMAA hydrogel cubes to hepsin-overexpressing tumor cells is further confirmed by a 3-10-fold higher particle internalization by hepsin-positive MCF-7 and SK-OV-3 compared to that of hepsin-negative PC-3 cells. This work provides a facile method to fabricate enhanced tumor-targeting carriers of submicrometer size and improves the general understanding of particle design parameters for targeted drug delivery.
中文翻译:

主动肿瘤细胞靶向的肽官能化水凝胶多维数据集。
生物活性靶向分子与纳米或微米尺寸药物载体的缀合是提高其治疗效率的关键策略。本文中,我们开发了具有表面共轭癌细胞靶向配体的pH和氧化还原敏感水凝胶颗粒,用于特异性靶向肿瘤和控制释放抗癌药阿霉素。通过在牺牲立方介孔核上进行多层聚合物组装,可生产700 nm和2μm的具有庚素靶向(IPLVVPL)表面肽的聚(甲基丙烯酸)(PMAA)水凝胶立方体。通过硫醇-胺反应将肽直接偶联至二硫键稳定的水凝胶不会损害结构完整性,亲水性,血清稳定性或pH /氧化还原敏感性,但会影响癌细胞的内在化。细胞摄取动力学和内在化的最终程度由细胞类型和水凝胶大小控制。由于配体-受体的识别,肽修饰显着促进了肝素阳性MCF-7细胞对700 nm水凝胶的吸收,但对2μmPMAA水凝胶的吸收影响可忽略不计。与肝素阴性的PC相比,肝素阳性的MCF-7和SK-OV-3的颗粒内在化程度高3-10倍,进一步证实了700 nm IPLVVPL-PMAA水凝胶立方体对过表达肝素的肿瘤细胞的选择性-3个单元格。这项工作提供了一种简便的方法来制造亚微米大小的增强的肿瘤靶向载体,并提高了对靶向药物递送的颗粒设计参数的一般理解。由于配体-受体的识别,肽修饰显着促进了肝素阳性MCF-7细胞对700 nm水凝胶的吸收,但对2μmPMAA水凝胶的吸收影响可忽略不计。与肝素阴性的PC相比,肝素阳性的MCF-7和SK-OV-3的颗粒内在化作用高3-10倍,进一步证实了700 nm IPLVVPL-PMAA水凝胶立方体对过表达肝素的肿瘤细胞的选择性-3个单元格。这项工作提供了一种简便的方法来制造亚微米大小的增强的肿瘤靶向载体,并提高了对靶向药物递送的颗粒设计参数的一般理解。由于配体-受体的识别,肽修饰显着促进了肝素阳性MCF-7细胞对700 nm水凝胶的吸收,但对2μmPMAA水凝胶的吸收影响可忽略不计。与肝素阴性的PC相比,肝素阳性的MCF-7和SK-OV-3的颗粒内在化作用高3-10倍,进一步证实了700 nm IPLVVPL-PMAA水凝胶立方体对过表达肝素的肿瘤细胞的选择性-3个单元格。这项工作提供了一种简便的方法来制造亚微米大小的增强的肿瘤靶向载体,并提高了对靶向药物递送的颗粒设计参数的一般理解。
更新日期:2018-08-31
中文翻译:

主动肿瘤细胞靶向的肽官能化水凝胶多维数据集。
生物活性靶向分子与纳米或微米尺寸药物载体的缀合是提高其治疗效率的关键策略。本文中,我们开发了具有表面共轭癌细胞靶向配体的pH和氧化还原敏感水凝胶颗粒,用于特异性靶向肿瘤和控制释放抗癌药阿霉素。通过在牺牲立方介孔核上进行多层聚合物组装,可生产700 nm和2μm的具有庚素靶向(IPLVVPL)表面肽的聚(甲基丙烯酸)(PMAA)水凝胶立方体。通过硫醇-胺反应将肽直接偶联至二硫键稳定的水凝胶不会损害结构完整性,亲水性,血清稳定性或pH /氧化还原敏感性,但会影响癌细胞的内在化。细胞摄取动力学和内在化的最终程度由细胞类型和水凝胶大小控制。由于配体-受体的识别,肽修饰显着促进了肝素阳性MCF-7细胞对700 nm水凝胶的吸收,但对2μmPMAA水凝胶的吸收影响可忽略不计。与肝素阴性的PC相比,肝素阳性的MCF-7和SK-OV-3的颗粒内在化程度高3-10倍,进一步证实了700 nm IPLVVPL-PMAA水凝胶立方体对过表达肝素的肿瘤细胞的选择性-3个单元格。这项工作提供了一种简便的方法来制造亚微米大小的增强的肿瘤靶向载体,并提高了对靶向药物递送的颗粒设计参数的一般理解。由于配体-受体的识别,肽修饰显着促进了肝素阳性MCF-7细胞对700 nm水凝胶的吸收,但对2μmPMAA水凝胶的吸收影响可忽略不计。与肝素阴性的PC相比,肝素阳性的MCF-7和SK-OV-3的颗粒内在化作用高3-10倍,进一步证实了700 nm IPLVVPL-PMAA水凝胶立方体对过表达肝素的肿瘤细胞的选择性-3个单元格。这项工作提供了一种简便的方法来制造亚微米大小的增强的肿瘤靶向载体,并提高了对靶向药物递送的颗粒设计参数的一般理解。由于配体-受体的识别,肽修饰显着促进了肝素阳性MCF-7细胞对700 nm水凝胶的吸收,但对2μmPMAA水凝胶的吸收影响可忽略不计。与肝素阴性的PC相比,肝素阳性的MCF-7和SK-OV-3的颗粒内在化作用高3-10倍,进一步证实了700 nm IPLVVPL-PMAA水凝胶立方体对过表达肝素的肿瘤细胞的选择性-3个单元格。这项工作提供了一种简便的方法来制造亚微米大小的增强的肿瘤靶向载体,并提高了对靶向药物递送的颗粒设计参数的一般理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号