Acta Biomaterialia ( IF 9.7 ) Pub Date : 2018-08-31 , DOI: 10.1016/j.actbio.2018.08.038 Yong Liu , Yijin Ren , Yuanfeng Li , Linzhu Su , Yumin Zhang , Fan Huang , Jinjian Liu , Jianfeng Liu , Theo G. van Kooten , Yingli An , Linqi Shi , Henny C. van der Mei , Henk J. Busscher
|
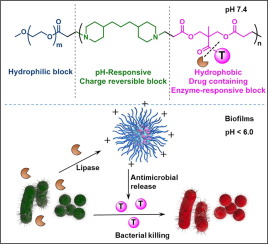
Conventional antimicrobials are becoming increasingly ineffective for treating bacterial infection due to the emergence of multi-drug resistant (MDR) pathogens. In addition, the biofilm-mode-of-growth of infecting bacteria impedes antimicrobial penetration in biofilms. Here, we report on poly(ethylene)glycol-poly(β-amino esters) (PEG-PAE) micelles with conjugated antimicrobials, that can uniquely penetrate biofilms, target themselves to bacterial cell surfaces once inside the low-pH environment of a biofilm and release conjugated antimicrobials through degradation of their ester-linkage with PAE by bacterial lipases. In vitro, PEG-PAE micelles with conjugated Triclosan (PEG-PAE-Triclosan) yielded no inadvertent leakage of their antimicrobial cargo and better killing of MDR Staphylococcus aureus, Escherichia coli and oral streptococcal biofilms than Triclosan in solution. In mice, PEG-PAE-Triclosan micelles with conjugated Triclosan yielded better eradication efficacy towards a MDR S. aureus-infection compared with Triclosan in solution and Triclosan-loaded micelles at equal Triclosan-equivalent concentrations. Ex vivo exposure of multi-species oral biofilms collected from orthodontic patients to PEG-PAE-Triclosan micelles, demonstrated effective bacterial killing at 30-40 fold lower Triclosan-equivalent concentrations than achieved by Triclosan in solution. Importantly, Streptococcus mutans, the main causative organism of dental caries, was preferentially killed by PEG-PAE-Triclosan micelles. Thus PEG-PAE-Triclosan micelles present a promising addendum to the decreasing armamentarium available to combat infection in diverse sites of the body.
Statement of Significance
pH-adaptive polymeric micelles with conjugated antimicrobials can efficiently eradicate infectious biofilms from diverse body sites in mice and men. An antimicrobial was conjugated through an ester-linkage to a poly(ethylene glycol) (PEG)/poly(β-amino ester) block copolymer to create micellar nanocarriers. Stable micelle structures were formed by the hydrophobic poly(β-amino ester) inner core and a hydrophilic PEG outer shell. Thus formed PEG-PAE-Triclosan micelles do not lose their antimicrobial cargo underway to an infection site through the blood circulation, but penetrate and accumulate in biofilms to release antimicrobials once inside a biofilm through degradation of its ester-linkage by bacterial lipases, to kill biofilm-embedded bacteria at lower antimicrobial concentrations than when applied in solution. PEG-PAE-Triclosan micelles effectively eradicate biofilms of multi-drug-resistant pathogens and oral bacteria, most notably highly cariogenic Streptococcus mutans, in mice and men respectively, and possess excellent clinical translation possibilities.
中文翻译:

在小鼠体内和人离体感染模型中评估了具有共轭抗微生物剂以根除病原性生物膜的纳米载体
由于出现多药耐药性(MDR)病原体,常规抗菌剂在治疗细菌感染方面变得越来越无效。另外,感染细菌的生物膜生长模式阻碍了抗菌剂在生物膜中的渗透。在这里,我们报道了具有共轭抗微生物剂的聚(乙二醇)-聚(β-氨基酯)(PEG-PAE)胶束,该胶束可以独特地渗透生物膜,一旦进入生物膜的低pH环境,其自身就可以靶向细菌细胞表面并通过细菌脂肪酶降解其与PAE的酯键来释放缀合的抗菌剂。在体外,结合有三氯生的PEG-PAE胶束(PEG-PAE-三氯生)不会无意泄漏其抗菌药物,并能更好地杀死金黄色葡萄球菌,大肠杆菌和口服链球菌生物膜均比三氯生溶液中的多。在小鼠中,与溶液中的三氯生和等量三氯生当量的三氯生负载的胶束相比,具有三氯生结合的PEG-PAE-三氯生胶束对金黄色葡萄球菌感染的根除效果更好。从正畸患者身上收集的多种物种口腔生物膜的体外暴露于PEG-PAE-Triclosan胶束,证明其有效杀灭细菌的三氯生当量浓度比溶液中三氯生低30-40倍。重要的是,变形链球菌龋齿的主要致病生物,是被PEG-PAE-三氯生胶束优先杀死的。因此,PEG-PAE-三氯生胶束为减少的武器库提供了有希望的补充品,可用于抵抗人体不同部位的感染。
重要声明
具有共轭抗微生物剂的,具有pH值的聚合胶束可以有效地根除小鼠和男性体内不同部位的感染性生物膜。通过酯键将抗菌剂与聚(乙二醇)(PEG)/聚(β-氨基酯)嵌段共聚物偶联,以形成胶束纳米载体。疏水的聚(β-氨基酯)内核和亲水的PEG外壳形成了稳定的胶束结构。如此形成的PEG-PAE-三氯生胶束不会通过血液循环流失到感染部位,而是渗透并积聚在生物膜中,一旦通过细菌脂肪酶降解其酯键,就可以在生物膜内部释放出抗菌剂,从而杀死细菌。生物膜包埋的细菌的抗菌浓度要低于溶液中的抗菌浓度。变形链球菌分别在小鼠和男性中,具有极好的临床翻译可能性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号