Acta Biomaterialia ( IF 9.7 ) Pub Date : 2018-08-31 , DOI: 10.1016/j.actbio.2018.08.033 Jihui Lee 1 , Shreedevi Arun Kumar 1 , Yong Yu Jhan 1 , Corey J Bishop 1
|
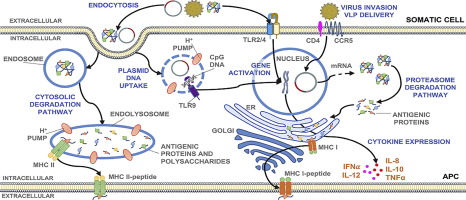
Engineering vaccine-based therapeutics for infectious diseases is highly challenging, as trial formulations are often found to be nonspecific, ineffective, thermally or hydrolytically unstable, and/or toxic. Vaccines have greatly improved the therapeutic landscape for treating infectious diseases and have significantly reduced the threat by therapeutic and preventative approaches. Furthermore, the advent of recombinant technologies has greatly facilitated growth within the vaccine realm by mitigating risks such as virulence reversion despite making the production processes more cumbersome. In addition, seroconversion can also be enhanced by recombinant technology through kinetic and nonkinetic approaches, which are discussed herein. Recombinant technologies have greatly improved both amino acid-based vaccines and DNA-based vaccines. A plateau of interest has been reached between 2001 and 2010 for the scientific community with regard to DNA vaccine endeavors. The decrease in interest may likely be attributed to difficulties in improving immunogenic properties associated with DNA vaccines, although there has been research demonstrating improvement and optimization to this end. Despite improvement, to the extent of our knowledge, there are currently no regulatory body-approved DNA vaccines for human use (four vaccines approved for animal use). This article discusses engineering DNA vaccines against infectious diseases while discussing advantages and disadvantages of each, with an emphasis on applications of these DNA vaccines.
Statement of Significance
This review paper summarizes the state of the engineered/recombinant DNA vaccine field, with a scope entailing “Engineering DNA vaccines against infectious diseases”. We endeavor to emphasize recent advances, recapitulating the current state of the field. In addition to discussing DNA therapeutics that have already been clinically translated, this review also examines current research developments, and the challenges thwarting further progression. Our review covers: recombinant DNA-based subunit vaccines; internalization and processing; enhancing immune protection via adjuvants; manufacturing and engineering DNA; the safety, stability and delivery of DNA vaccines or plasmids; controlling gene expression using plasmid engineering and gene circuits; overcoming immunogenic issues; and commercial successes. We hope that this review will inspire further research in DNA vaccine development.
中文翻译:

针对传染病的工程DNA疫苗。
基于工程疫苗的传染病治疗剂具有很高的挑战性,因为通常发现试验制剂是非特异性的,无效的,热或水解不稳定的和/或有毒的。疫苗极大地改善了治疗传染病的治疗前景,并通过治疗和预防手段大大降低了威胁。此外,尽管降低了生产过程的麻烦,但重组技术的出现极大地促进了疫苗领域内的生长,尽管它减轻了毒力回复等风险,但仍大大促进了疫苗领域的发展。另外,血清转化还可以通过重组技术通过动力学和非动力学方法来增强,这在本文中进行了讨论。重组技术极大地改善了基于氨基酸的疫苗和基于DNA的疫苗。在2001年至2010年之间,科学界对DNA疫苗的研究已经达到了一个感兴趣的平台。尽管已有研究表明为此目的进行了改进和优化,但兴趣的下降很可能归因于与DNA疫苗相关的免疫原性的改善。就我们所知,尽管有所改进,但目前尚无经监管机构批准的供人类使用的DNA疫苗(已批准将四种疫苗用于动物用途)。本文讨论了针对传染病的工程DNA疫苗,同时讨论了每种疫苗的优缺点,并着重介绍了这些DNA疫苗的应用。尽管已有研究表明为此目的进行了改进和优化,但兴趣的下降很可能归因于与DNA疫苗相关的免疫原性的改善。就我们所知,尽管有所改进,但目前尚无经监管机构批准的供人类使用的DNA疫苗(已批准将四种疫苗用于动物用途)。本文讨论了针对传染病的工程DNA疫苗,同时讨论了每种疫苗的优缺点,并着重介绍了这些DNA疫苗的应用。尽管已有研究表明为此目的进行了改进和优化,但兴趣的下降很可能归因于与DNA疫苗相关的免疫原性的改善。就我们所知,尽管有所改进,但目前尚无经监管机构批准的供人类使用的DNA疫苗(已批准将四种疫苗用于动物用途)。本文讨论了针对传染病的工程DNA疫苗,同时讨论了每种疫苗的优缺点,并着重介绍了这些DNA疫苗的应用。目前,尚无经监管机构批准的供人类使用的DNA疫苗(四种疫苗已获批准用于动物用途)。本文讨论了针对传染病的工程DNA疫苗,同时讨论了每种疫苗的优缺点,并着重介绍了这些DNA疫苗的应用。目前,尚无经监管机构批准的供人类使用的DNA疫苗(四种疫苗已获批准用于动物用途)。本文讨论了针对传染病的工程DNA疫苗,同时讨论了每种疫苗的优缺点,并着重介绍了这些DNA疫苗的应用。
重要声明
这篇综述文章总结了工程化/重组DNA疫苗领域的现状,其范围为“针对传染病的工程化DNA疫苗”。我们努力强调最新进展,概括了该领域的当前状况。除了讨论已经进行临床翻译的DNA治疗方法外,本综述还探讨了当前的研究进展以及阻碍进一步发展的挑战。我们的综述涵盖:基于重组DNA的亚单位疫苗;内部化和处理;通过佐剂增强免疫保护;制造和工程DNA;DNA疫苗或质粒的安全性,稳定性和递送;使用质粒工程和基因电路控制基因表达;克服免疫原性问题;和商业上的成功。


























 京公网安备 11010802027423号
京公网安备 11010802027423号