当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct Measurement of High-Temperature Rate Constants of the Thermal Decomposition of Dimethoxymethane, a Shock Tube and Modeling Study
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-08-30 00:00:00 , DOI: 10.1021/acs.jpca.8b06558 Sebastian Peukert 1 , Paul Sela 1 , Damien Nativel 1 , Jürgen Herzler 1 , Mustapha Fikri 1 , Christof Schulz 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-08-30 00:00:00 , DOI: 10.1021/acs.jpca.8b06558 Sebastian Peukert 1 , Paul Sela 1 , Damien Nativel 1 , Jürgen Herzler 1 , Mustapha Fikri 1 , Christof Schulz 1
Affiliation
|
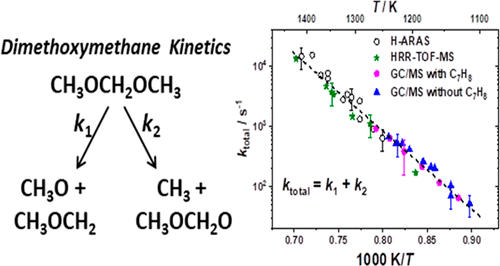
Shock-tube experiments have been performed to investigate the thermal decomposition of the oxygenated hydrocarbon dimethoxymethane (DMM; CH3OCH2OCH3). The primary initial reaction channels of DMM decomposition are considered to be the two bond fissions: CH3OCH2OCH3 → CH3O + CH2OCH3 (1) and CH3OCH2OCH3 → CH3 + OCH2OCH3 (2). In the present work, two shock-tube facilities and three different detection techniques have been combined: Behind reflected shock waves, we have carried out time-resolved measurements of (i) the formation of H atoms using the highly sensitive H-ARAS (Atomic Resonance Absorption Spectrometry) technique and (ii) the depletion of the DMM reactant by high-repetition-rate time-of-flight mass spectrometry (HRR-TOF-MS). In addition, (iii) the temperature-dependent composition of stable reaction products was measured in single-pulse shock-tube experiments via gas chromatography (GC/MS). The experiments span a temperature range of 1100–1430 K, a pressure range of 1.2–2.5 bar, and initial reactant mole fractions from 0.5 ppm (for H-ARAS experiments) up to 10 000 ppm (for HRR-TOF-MS experiments). Experimental rate constants ktotal, ktotal = k1 + k2, obtained from these three completely different methods were in excellent agreement among each other, i.e., deviations are within ±30–40%, and they can be well represented by the Arrhenius expression ktotal(T) = 1013.28±0.27 exp(−247.90 ± 6.36 kJ mol–1/RT) s–1 (valid over the 1100–1400 K temperature and the 1.2–2.5 bar pressure range). By replacing the respective ktotal values used in a recently published DMM chemical kinetics combustion mechanism (Vermeire et al. Combust. Flame2018, 190, 270–283), it was also possible to successfully reproduce measured product distributions.
中文翻译:

直接测量二甲氧基甲烷热分解的高温速率常数,激波管和建模研究
已经进行了激波管实验,以研究含氧烃二甲氧基甲烷(DMM; CH 3 OCH 2 OCH 3)的热分解。DMM分解的主要初始反应通道被认为是两个键裂变:CH 3 OCH 2 OCH 3 →CH 3 O + CH 2 OCH 3(1)和CH 3 OCH 2 OCH 3 →CH 3 + OCH 2 OCH 3(2)。在目前的工作中,将两个激波管设施和三种不同的检测技术结合在一起:在反射的激波后面,我们已经进行了时间分辨测量(i)使用高度敏感的H-ARAS(原子)形成H原子。共振吸收光谱法)(ii)通过高重复速率飞行时间质谱(HRR-TOF-MS)去除DMM反应物。另外,(iii)通过气相色谱法(GC / MS)在单脉冲冲击管实验中测量了稳定反应产物的温度依赖性组成。实验的温度范围为1100-1430 K,压力范围为1.2-2.5 bar,初始反应物摩尔分数从0.5 ppm(对于H-ARAS实验)到10000 ppm(对于HRR-TOF-MS实验) 。实验速率常数k通过这三种完全不同的方法获得的total,k total = k 1 + k 2,彼此之间具有极好的一致性,即偏差在±30–40%之内,并且可以用Arrhenius表达式k total很好地表示(T)= 10 13.28±0.27 exp(−247.90±6.36 kJ mol –1 / RT)s –1(在1100–1400 K温度和1.2–2.5 bar压力范围内有效)。通过替换最近发表的DMM化学动力学燃烧机理中使用的各个k总值(Vermeire等人,燃烧 火焰2018,190,270-283),也有人可能成功地再现测定产物分布。
更新日期:2018-08-30
中文翻译:

直接测量二甲氧基甲烷热分解的高温速率常数,激波管和建模研究
已经进行了激波管实验,以研究含氧烃二甲氧基甲烷(DMM; CH 3 OCH 2 OCH 3)的热分解。DMM分解的主要初始反应通道被认为是两个键裂变:CH 3 OCH 2 OCH 3 →CH 3 O + CH 2 OCH 3(1)和CH 3 OCH 2 OCH 3 →CH 3 + OCH 2 OCH 3(2)。在目前的工作中,将两个激波管设施和三种不同的检测技术结合在一起:在反射的激波后面,我们已经进行了时间分辨测量(i)使用高度敏感的H-ARAS(原子)形成H原子。共振吸收光谱法)(ii)通过高重复速率飞行时间质谱(HRR-TOF-MS)去除DMM反应物。另外,(iii)通过气相色谱法(GC / MS)在单脉冲冲击管实验中测量了稳定反应产物的温度依赖性组成。实验的温度范围为1100-1430 K,压力范围为1.2-2.5 bar,初始反应物摩尔分数从0.5 ppm(对于H-ARAS实验)到10000 ppm(对于HRR-TOF-MS实验) 。实验速率常数k通过这三种完全不同的方法获得的total,k total = k 1 + k 2,彼此之间具有极好的一致性,即偏差在±30–40%之内,并且可以用Arrhenius表达式k total很好地表示(T)= 10 13.28±0.27 exp(−247.90±6.36 kJ mol –1 / RT)s –1(在1100–1400 K温度和1.2–2.5 bar压力范围内有效)。通过替换最近发表的DMM化学动力学燃烧机理中使用的各个k总值(Vermeire等人,燃烧 火焰2018,190,270-283),也有人可能成功地再现测定产物分布。



























 京公网安备 11010802027423号
京公网安备 11010802027423号