当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New oxidovanadium(iv) complex with a BIAN ligand: synthesis, structure, redox properties and catalytic activity†
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-08-30 00:00:00 , DOI: 10.1039/c8nj03358g Iakov S. Fomenko 1, 2, 3 , Artem L. Gushchin 1, 2, 3, 4, 5 , Lidia S. Shul’pina 3, 6, 7, 8 , Nikolay S. Ikonnikov 3, 6, 7, 8 , Pavel A. Abramov 1, 2, 3 , Nikolay F. Romashev 1, 2, 3, 4, 5 , Artem S. Poryvaev 3, 4, 5, 5, 9 , Alena M. Sheveleva 3, 4, 5, 5, 9 , Artem S. Bogomyakov 3, 5, 9 , Nikita Y. Shmelev 1, 2, 3, 4, 5 , Matvey V. Fedin 3, 5, 9 , Georgiy B. Shul’pin 3, 8, 10, 11, 12 , Maxim N. Sokolov 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-08-30 00:00:00 , DOI: 10.1039/c8nj03358g Iakov S. Fomenko 1, 2, 3 , Artem L. Gushchin 1, 2, 3, 4, 5 , Lidia S. Shul’pina 3, 6, 7, 8 , Nikolay S. Ikonnikov 3, 6, 7, 8 , Pavel A. Abramov 1, 2, 3 , Nikolay F. Romashev 1, 2, 3, 4, 5 , Artem S. Poryvaev 3, 4, 5, 5, 9 , Alena M. Sheveleva 3, 4, 5, 5, 9 , Artem S. Bogomyakov 3, 5, 9 , Nikita Y. Shmelev 1, 2, 3, 4, 5 , Matvey V. Fedin 3, 5, 9 , Georgiy B. Shul’pin 3, 8, 10, 11, 12 , Maxim N. Sokolov 1, 2, 3, 4, 5
Affiliation
|
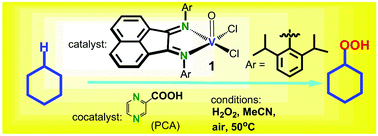
Reaction of VCl3 with bis[N-(2,6-diisopropylphenyl)imino]acenaphthene (dpp-bian) in air afforded [VOCl2(dpp-bian)] (1). The complex was characterized by IR and UV-vis spectroscopies and elemental analysis. The crystal structure of 1 was determined by X-ray diffraction (XRD) analysis. The vanadium atom is in a square-pyramidal OCl2N4 coordination environment. The cyclic voltammogram (CV) in dichloromethane reveals an irreversible oxidation process at +1.40 V (vs. Ag/AgCl) assigned to the V(IV)/V(V) couple, and two consecutive quasi-reversible one-electron reduction processes at −0.32 V and −1.05 V (vs. Ag/AgCl), respectively, assigned to the bian/bian−/˙ and bian−/˙/bian2− couples, followed by irreversible reduction at −1.6 V (vs. Ag/AgCl). The EPR spectrum of 1 in toluene shows a single 8-line signal typical for oxidovanadium(IV) complexes with d1 configuration. The magnetic behavior of 1 confirms the presence of one unpaired electron (μeff (330 K) = 1.67 μB), and the isolation of the paramagnetic centers. Application of 1 to oxidation of alkanes documented high catalytic activity under mild conditions. The kinetics and selectivity of alkane oxygenation by the 1/H2O2 and 1/PCA/H2O2 systems (PCA is pyrazine-2-carboxylic acid) were studied. The reaction is more efficient in the presence of PCA.
中文翻译:

具有BIAN配体的新型氧化钒(iv)配合物:合成,结构,氧化还原性质和催化活性†
VCl 3与双[ N-(2,6-二异丙基苯基)亚氨基] ac(dpp-bian)在空气中反应,得到[VOCl 2(dpp-bian)](1)。该复合物通过IR和UV-可见光谱法和元素分析来表征。通过X射线衍射(XRD)分析确定1的晶体结构。钒原子处于方形金字塔形的OCl 2 N 4配位环境中。在二氯甲烷中的循环伏安图(CV)揭示了一种不可逆的氧化工艺在1.40 V(相对于分配给V(银/氯化银)IV)/ V(V)耦合和分别在-0.32 V和-1.05 V(相对于Ag / AgCl)的两个连续的准可逆单电子还原过程分配给bian / bian- / ˙和bian- / ˙/ bian 2-耦合,然后在-1.6 V时不可逆还原(相对于Ag / AgCl)。甲苯中的1的EPR谱图显示了具有d 1构型的氧化钒(IV)配合物的单个8线信号。的磁性行为1次确认一个不成对电子的存在(μ EFF(330 K)= 1.67 μ乙),以及顺磁中心的隔离。1在烷烃氧化中的应用证明在温和的条件下具有很高的催化活性。研究了通过1 / H 2 O 2和1 / PCA / H 2 O 2系统(PCA为吡嗪-2-羧酸)进行烷烃氧化的动力学和选择性。在PCA存在下,该反应更有效。
更新日期:2018-08-30
中文翻译:

具有BIAN配体的新型氧化钒(iv)配合物:合成,结构,氧化还原性质和催化活性†
VCl 3与双[ N-(2,6-二异丙基苯基)亚氨基] ac(dpp-bian)在空气中反应,得到[VOCl 2(dpp-bian)](1)。该复合物通过IR和UV-可见光谱法和元素分析来表征。通过X射线衍射(XRD)分析确定1的晶体结构。钒原子处于方形金字塔形的OCl 2 N 4配位环境中。在二氯甲烷中的循环伏安图(CV)揭示了一种不可逆的氧化工艺在1.40 V(相对于分配给V(银/氯化银)IV)/ V(V)耦合和分别在-0.32 V和-1.05 V(相对于Ag / AgCl)的两个连续的准可逆单电子还原过程分配给bian / bian- / ˙和bian- / ˙/ bian 2-耦合,然后在-1.6 V时不可逆还原(相对于Ag / AgCl)。甲苯中的1的EPR谱图显示了具有d 1构型的氧化钒(IV)配合物的单个8线信号。的磁性行为1次确认一个不成对电子的存在(μ EFF(330 K)= 1.67 μ乙),以及顺磁中心的隔离。1在烷烃氧化中的应用证明在温和的条件下具有很高的催化活性。研究了通过1 / H 2 O 2和1 / PCA / H 2 O 2系统(PCA为吡嗪-2-羧酸)进行烷烃氧化的动力学和选择性。在PCA存在下,该反应更有效。


























 京公网安备 11010802027423号
京公网安备 11010802027423号