当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective synthesis of axially chiral vinyl arenes through palladium-catalyzed C–H olefination†
Chemical Communications ( IF 4.9 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1039/c8cc05555f Qiao-Ying Sun 1, 2, 3, 4 , Wei-Yang Ma 1, 2, 3, 4 , Ke-Fang Yang 1, 2, 3, 4 , Jian Cao 1, 2, 3, 4 , Zhan-Jiang Zheng 1, 2, 3, 4 , Zheng Xu 1, 2, 3, 4 , Yu-Ming Cui 1, 2, 3, 4 , Li-Wen Xu 1, 2, 3, 4
Chemical Communications ( IF 4.9 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1039/c8cc05555f Qiao-Ying Sun 1, 2, 3, 4 , Wei-Yang Ma 1, 2, 3, 4 , Ke-Fang Yang 1, 2, 3, 4 , Jian Cao 1, 2, 3, 4 , Zhan-Jiang Zheng 1, 2, 3, 4 , Zheng Xu 1, 2, 3, 4 , Yu-Ming Cui 1, 2, 3, 4 , Li-Wen Xu 1, 2, 3, 4
Affiliation
|
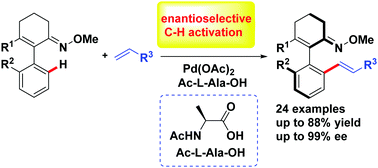
A palladium-catalyzed ketoxime-chelation-assisted enantioselective C–H olefination of 2-arylcyclohex-2-enone oxime ether with a wide range of olefins as coupling partners was developed. Employing ketoxime ether as an efficient directing group, a variety of axially chiral vinyl arenes was synthesized by Pd(II)-catalyzed C–H functionalization with excellent enantioselectivities (96 → 99% ee) under mild conditions.
中文翻译:

通过钯催化的CH-H烯化反应,对映选择性地合成轴向手性乙烯基芳烃†
开发了钯催化的酮肟肟螯合辅助的2-芳基环己-2-烯酮肟醚的对映选择性C-H烯烃化反应,该反应中使用了多种烯烃作为偶联伙伴。利用酮肟醚作为有效的导向基团,在温和的条件下,通过Pd(II)催化的C–H官能化,具有优异的对映选择性(96→99%ee),合成了多种轴向手性乙烯基芳烃。
更新日期:2018-08-29
中文翻译:

通过钯催化的CH-H烯化反应,对映选择性地合成轴向手性乙烯基芳烃†
开发了钯催化的酮肟肟螯合辅助的2-芳基环己-2-烯酮肟醚的对映选择性C-H烯烃化反应,该反应中使用了多种烯烃作为偶联伙伴。利用酮肟醚作为有效的导向基团,在温和的条件下,通过Pd(II)催化的C–H官能化,具有优异的对映选择性(96→99%ee),合成了多种轴向手性乙烯基芳烃。



























 京公网安备 11010802027423号
京公网安备 11010802027423号