当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facile one-pot synthesis of spirooxindole-pyrrolidine derivatives and their antimicrobial and acetylcholinesterase inhibitory activities†
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1039/c8nj03813a Ying Huang 1, 2, 3, 4 , Wei Min 4, 5, 6, 7 , Qiu-Wen Wu 2, 4, 8, 9 , Jing Sun 1, 2, 3, 4 , Da-Hua Shi 4, 5, 6, 7 , Chao-Guo Yan 1, 2, 3, 4
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1039/c8nj03813a Ying Huang 1, 2, 3, 4 , Wei Min 4, 5, 6, 7 , Qiu-Wen Wu 2, 4, 8, 9 , Jing Sun 1, 2, 3, 4 , Da-Hua Shi 4, 5, 6, 7 , Chao-Guo Yan 1, 2, 3, 4
Affiliation
|
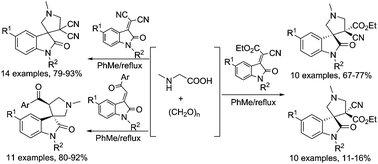
A library of novel spirooxindole-pyrrolidine derivatives were facilely synthesized via 1,3-dipolar cycloaddition of azomethine ylide generated from sarcosine and paraformaldehyde with various 3-methyleneoxindolines. The relative configuration of obtained spiro[indoline-3,3′-pyrrolidines] was successfully elucidated by determination of nine single-crystal structures. The antimicrobial activities and the inhibitory activity towards acetylcholinesterase of the synthesized spiro compounds were also preliminarily evaluated, in which the spiro[indoline-3,3′-pyrrolidine] 4d exhibited the most potent activity with an IC50 value of 69.07 ± 10.99 μM against acetylcholinesterase (AChE).
中文翻译:

便捷的一锅合成螺环吲哚-吡咯烷衍生物及其抑菌和乙酰胆碱酯酶抑制活性†
通过由肌氨酸和多聚甲醛产生的甲亚胺基内酯的1,3-偶极环加成反应与各种3-亚甲基二氢吲哚合成新的螺氧杂吲哚-吡咯烷衍生物文库。通过测定九个单晶结构,成功阐明了螺[吲哚啉-3,3'-吡咯烷]的相对构型。还初步评估了合成的螺化合物的抗菌活性和对乙酰胆碱酯酶的抑制活性,其中螺[吲哚啉-3,3'-吡咯烷] 4d表现出最强的活性,其IC 50值为69.07±10.99μM。乙酰胆碱酯酶(AChE)。
更新日期:2018-08-29
中文翻译:

便捷的一锅合成螺环吲哚-吡咯烷衍生物及其抑菌和乙酰胆碱酯酶抑制活性†
通过由肌氨酸和多聚甲醛产生的甲亚胺基内酯的1,3-偶极环加成反应与各种3-亚甲基二氢吲哚合成新的螺氧杂吲哚-吡咯烷衍生物文库。通过测定九个单晶结构,成功阐明了螺[吲哚啉-3,3'-吡咯烷]的相对构型。还初步评估了合成的螺化合物的抗菌活性和对乙酰胆碱酯酶的抑制活性,其中螺[吲哚啉-3,3'-吡咯烷] 4d表现出最强的活性,其IC 50值为69.07±10.99μM。乙酰胆碱酯酶(AChE)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号