当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chiral Amine‐Catalyzed Stereoselective [4+2] Annulations of Alkenyl Thiazolones and Aliphatic Aldehydes via a Step‐Wise Mechanism
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-10-07 , DOI: 10.1002/adsc.201800806 Qing-Song Dai 1 , Xiang Zhang 1 , Kai-Chuan Yang 1 , Mao-Hua Li 1 , Jie Yang 1 , Qing-Zhu Li 1 , Xin Feng 1 , Bo Han 2 , Jun-Long Li 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-10-07 , DOI: 10.1002/adsc.201800806 Qing-Song Dai 1 , Xiang Zhang 1 , Kai-Chuan Yang 1 , Mao-Hua Li 1 , Jie Yang 1 , Qing-Zhu Li 1 , Xin Feng 1 , Bo Han 2 , Jun-Long Li 1
Affiliation
|
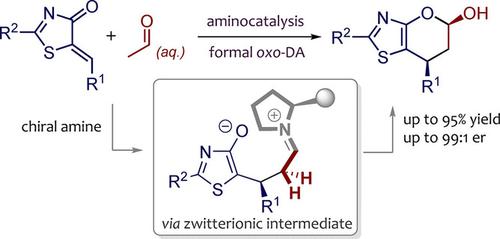
An efficient diastereo‐ and enantioselective formal oxo‐Diels‐Alder reaction of enolizable aliphatic aldehydes and 5‐alkenyl thiazolones was developed through chiral amine catalysis. With a robust enamine activation strategy, both simple aliphatic aldehydes and a challenging aqueous acetaldehyde could participate well as competent dienophiles in the reaction. A variety of thiazole‐fused dihydropyran derivatives were facilely produced in up to 99% yield with up to >99:1 e.r. under mild conditions. Interestingly, a high dosage of the inexpensive aqueous acetaldehyde in a similar catalytic system could lead to a densely functionalized thiazolone product through a three‐component cascade reaction, suggesting a step‐wise mechanism for the established [4+2] annulation process.
中文翻译:

手性胺催化的立体选择性[4 + 2]环烯基噻唑酮和脂族醛的逐步明智机理
通过手性胺催化,开发了可烯化的脂族醛和5-烯基噻唑酮的高效非对映和对映选择性形式氧代-狄尔斯-阿尔德反应。有了强大的烯胺活化策略,简单的脂族醛和具有挑战性的乙醛水溶液都可以作为有效的亲二烯体参与反应。在温和条件下,可以轻松地以高达99%的收率轻松生产各种噻唑稠合的二氢吡喃衍生物。有趣的是,在类似的催化系统中,高剂量的廉价乙醛水溶液可能会通过三组分级联反应导致稠密地官能化的噻唑酮产物,这为已建立的[4 + 2]环化过程提供了逐步的机制。
更新日期:2018-10-07
中文翻译:

手性胺催化的立体选择性[4 + 2]环烯基噻唑酮和脂族醛的逐步明智机理
通过手性胺催化,开发了可烯化的脂族醛和5-烯基噻唑酮的高效非对映和对映选择性形式氧代-狄尔斯-阿尔德反应。有了强大的烯胺活化策略,简单的脂族醛和具有挑战性的乙醛水溶液都可以作为有效的亲二烯体参与反应。在温和条件下,可以轻松地以高达99%的收率轻松生产各种噻唑稠合的二氢吡喃衍生物。有趣的是,在类似的催化系统中,高剂量的廉价乙醛水溶液可能会通过三组分级联反应导致稠密地官能化的噻唑酮产物,这为已建立的[4 + 2]环化过程提供了逐步的机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号