当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biochemical and Structural Characterization of TtnD, a Prenylated FMN-Dependent Decarboxylase from the Tautomycetin Biosynthetic Pathway
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-08-28 00:00:00 , DOI: 10.1021/acschembio.8b00673 Thibault Annaval 1 , Lu Han , Jeffrey D. Rudolf 1 , Guangbo Xie 1 , Dong Yang 1 , Chin-Yuan Chang 1 , Ming Ma 1 , Ivana Crnovcic 1 , Mitchell D. Miller , Jayashree Soman , Weijun Xu , George N. Phillips , Ben Shen 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-08-28 00:00:00 , DOI: 10.1021/acschembio.8b00673 Thibault Annaval 1 , Lu Han , Jeffrey D. Rudolf 1 , Guangbo Xie 1 , Dong Yang 1 , Chin-Yuan Chang 1 , Ming Ma 1 , Ivana Crnovcic 1 , Mitchell D. Miller , Jayashree Soman , Weijun Xu , George N. Phillips , Ben Shen 1
Affiliation
|
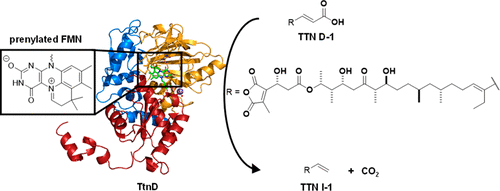
Tautomycetin (TTN) is a polyketide natural product featuring a terminal alkene. Functional characterization of the genes within the ttn gene cluster from Streptomyces griseochromogenes established the biosynthesis of the TTN polyketide backbone, its dialkylmaleic anhydride moiety, the coupling of the two moieties to form the nascent intermediate TTN F-1, and the tailoring steps converting TTN F-1 to TTN. Here, we report biochemical and structural characterization of TtnD, a prenylated FMN (prFMN)-dependent decarboxylase belonging to the UbiD family that catalyzes the penultimate step of TTN biosynthesis. TtnD catalyzes decarboxylation of TTN D-1 to TTN I-1, utilizing prFMN as a cofactor generated by the TtnC flavin prenyltransferase; both TtnD and TtnC are encoded within the ttn biosynthetic gene cluster. TtnD exhibits substrate promiscuity but accepts only TTN D-1 congeners that feature an α,β-unsaturated acid, supporting the [3+2] cycloaddition mechanism during catalysis that requires the double bond of an α,β-unsaturated acid substrate. TtnD shares a similar overall structure with other members of the UbiD family but forms a homotetramer in solution. Each protomer is composed of three domains with the active site located between the middle and C-terminal domains; R169-E272-E277, constituting the catalytic triad, and E228, involved in Mn(II)-mediated binding of prFMN, were confirmed by site-directed mutagenesis. TtnD represents the first example of a prFMN-dependent decarboxylase involved in polyketide biosynthesis, expanding the substrate scope of the UbiD family of decarboxylases beyond simple aromatic and cinnamic acids. TtnD and its homologues are widespread in nature and could be exploited as biocatalysts for organic synthesis.
中文翻译:

TtnD的生化和结构表征,TtnD是一种互变异构菌素生物合成途径中依赖于戊二烯的FMN脱羧酶。
互变霉素(TTN)是具有末端烯烃的聚酮化合物天然产物。该内的基因的功能表征TTN从基因簇链霉菌griseochromogenes建立的TTN聚酮主链的生物合成,其dialkylmaleic酸酐部分,这两个部分的耦合,以形成新生中间TTN F-1,和剪裁步骤转换TTN˚F -1至TTN。在这里,我们报告TtnD的生化和结构表征,TtnD是属于UbiD家族的催化TTN生物合成的倒数第二步的依赖于戊烯酰的FMN(prFMN)依赖性脱羧酶。TtnD利用prFMN作为由TtnC黄素异戊二烯基转移酶产生的辅因子,催化TTN D-1脱羧为TTN I-1;TtnD和TtnC都在n生物合成基因簇。TtnD表现出底物混杂,但仅接受具有α,β-不饱和酸特征的TTN D-1同系物,在催化过程中支持[3 + 2]环加成机理,这需要α,β-不饱和酸底物的双键。TtnD与UbiD家族的其他成员具有相似的总体结构,但在溶液中形成了同四聚体。每个启动子由三个域组成,其活性位点位于中间和C端域之间。通过定点诱变证实了构成催化三联体的R169-E272-E277和参与Mn(II)介导的prFMN结合的E228。TtnD代表参与聚酮化合物生物合成的prFMN依赖性脱羧酶的第一个例子,将UbiD脱羧酶家族的底物范围扩展到简单的芳族和肉桂酸之外。TtnD及其同系物在自然界很普遍,可以用作有机合成的生物催化剂。
更新日期:2018-08-28
中文翻译:

TtnD的生化和结构表征,TtnD是一种互变异构菌素生物合成途径中依赖于戊二烯的FMN脱羧酶。
互变霉素(TTN)是具有末端烯烃的聚酮化合物天然产物。该内的基因的功能表征TTN从基因簇链霉菌griseochromogenes建立的TTN聚酮主链的生物合成,其dialkylmaleic酸酐部分,这两个部分的耦合,以形成新生中间TTN F-1,和剪裁步骤转换TTN˚F -1至TTN。在这里,我们报告TtnD的生化和结构表征,TtnD是属于UbiD家族的催化TTN生物合成的倒数第二步的依赖于戊烯酰的FMN(prFMN)依赖性脱羧酶。TtnD利用prFMN作为由TtnC黄素异戊二烯基转移酶产生的辅因子,催化TTN D-1脱羧为TTN I-1;TtnD和TtnC都在n生物合成基因簇。TtnD表现出底物混杂,但仅接受具有α,β-不饱和酸特征的TTN D-1同系物,在催化过程中支持[3 + 2]环加成机理,这需要α,β-不饱和酸底物的双键。TtnD与UbiD家族的其他成员具有相似的总体结构,但在溶液中形成了同四聚体。每个启动子由三个域组成,其活性位点位于中间和C端域之间。通过定点诱变证实了构成催化三联体的R169-E272-E277和参与Mn(II)介导的prFMN结合的E228。TtnD代表参与聚酮化合物生物合成的prFMN依赖性脱羧酶的第一个例子,将UbiD脱羧酶家族的底物范围扩展到简单的芳族和肉桂酸之外。TtnD及其同系物在自然界很普遍,可以用作有机合成的生物催化剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号