当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Single Route to Mammalian N‐Glycans Substituted with Core Fucose and Bisecting GlcNAc
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-10-11 , DOI: 10.1002/anie.201807742 Thomas Luber 1 , Mathäus Niemietz 1 , Theodoros Karagiannis 1 , Manuel Mönnich 1 , Dimitri Ott 1 , Lukas Perkams 1 , Janika Walcher 1 , Lukas Berger 1 , Matthias Pischl 1 , Markus Weishaupt 1 , Steffen Eller 1 , Joanna Hoffman 1 , Carlo Unverzagt 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-10-11 , DOI: 10.1002/anie.201807742 Thomas Luber 1 , Mathäus Niemietz 1 , Theodoros Karagiannis 1 , Manuel Mönnich 1 , Dimitri Ott 1 , Lukas Perkams 1 , Janika Walcher 1 , Lukas Berger 1 , Matthias Pischl 1 , Markus Weishaupt 1 , Steffen Eller 1 , Joanna Hoffman 1 , Carlo Unverzagt 1
Affiliation
|
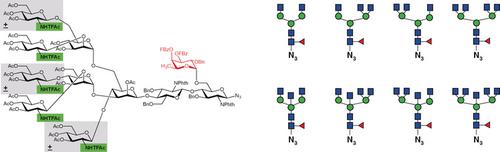
The occurrence of α1,6‐linked core fucose on the N‐glycans of mammalian glycoproteins is involved in tumor progression and reduces the bioactivity of antibodies in antibody‐dependent cell‐mediated cytotoxicity (ADCC). Since core‐fucosylated N‐glycans are difficult to isolate from natural sources, only chemical or enzymatic synthesis can provide the desired compounds for biological studies. A general drawback of chemical α‐fucosylation is that the chemical assembly of α1,6‐linked fucosides is not stereospecific. A robust and general method for the α‐selective fucosylation of acceptors with primary hydroxy groups in α/β ratios exceeding 99:1 was developed. The high selectivities result from the interplay of an optimized protecting group pattern of the fucosyl donors in combination with the activation principle and the reaction conditions. Selective deprotection yielded versatile azides of all mammalian complex‐type core‐fucosylated N‐glycans with 2‐4 antennae and optional bisecting GlcNAc.
中文翻译:

用核心岩藻糖和二等分GlcNAc替代哺乳动物N-聚糖的单一路线
哺乳动物糖蛋白的N-聚糖上α1,6连接的核心岩藻糖的存在与肿瘤进展有关,并降低了抗体依赖性细胞介导的细胞毒性(ADCC)中抗体的生物活性。由于核心岩藻糖基化的N-聚糖很难从天然来源中分离出来,因此只有化学或酶促合成才能为生物学研究提供所需的化合物。化学α-岩藻糖基化的一般缺点是,α1,6-连接岩藻糖苷的化学组装不是立体特异性的。开发了一种鲁棒且通用的方法,用于以α/β比超过99:1的具有伯羟基的受体进行α-选择性岩藻糖基化。岩藻糖基供体的优化保护基团图案与激活原理和反应条件相结合,相互作用高。
更新日期:2018-10-11
中文翻译:

用核心岩藻糖和二等分GlcNAc替代哺乳动物N-聚糖的单一路线
哺乳动物糖蛋白的N-聚糖上α1,6连接的核心岩藻糖的存在与肿瘤进展有关,并降低了抗体依赖性细胞介导的细胞毒性(ADCC)中抗体的生物活性。由于核心岩藻糖基化的N-聚糖很难从天然来源中分离出来,因此只有化学或酶促合成才能为生物学研究提供所需的化合物。化学α-岩藻糖基化的一般缺点是,α1,6-连接岩藻糖苷的化学组装不是立体特异性的。开发了一种鲁棒且通用的方法,用于以α/β比超过99:1的具有伯羟基的受体进行α-选择性岩藻糖基化。岩藻糖基供体的优化保护基团图案与激活原理和反应条件相结合,相互作用高。



























 京公网安备 11010802027423号
京公网安备 11010802027423号