当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Engineering a Glucosamine-6-phosphate Responsive glmS Ribozyme Switch Enables Dynamic Control of Metabolic Flux in Bacillus subtilis for Overproduction of N-Acetylglucosamine
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1021/acssynbio.8b00196 Tengfei Niu 1, 2 , Yanfeng Liu 1, 2 , Jianghua Li 1, 2 , Mattheos Koffas 3, 4 , Guocheng Du 1, 2 , Hal S. Alper 5, 6 , Long Liu 1, 2
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1021/acssynbio.8b00196 Tengfei Niu 1, 2 , Yanfeng Liu 1, 2 , Jianghua Li 1, 2 , Mattheos Koffas 3, 4 , Guocheng Du 1, 2 , Hal S. Alper 5, 6 , Long Liu 1, 2
Affiliation
|
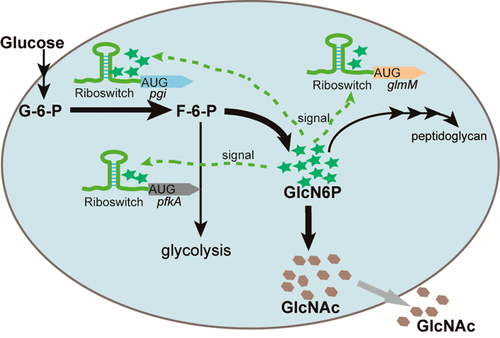
Bacillus subtilis is a typical industrial microorganism and is widely used in industrial biotechnology, particularly for nutraceutical production. There are many studies on the static metabolic engineering of B. subtilis, whereas there are few reports on dynamic metabolic engineering due to the lack of appropriate elements. Here, we established a dynamic reprogramming strategy for reconstructing metabolic networks in B. subtilis, using a typical nutraceutical, N-acetylglucosamine (GlcNAc), as a model product and the glmS (encoding glucosamine-6-phosphate synthase) ribozyme as an engineering element. First, a trp terminator was introduced to effectively release the glmS ribozyme feedback inhibition. Further, we engineered the native glucosamine-6-phosphate (GlcN6P) responsive glmS ribozyme switch to dynamically control the metabolic flux in B. subtilis for overproduction of GlcNAc. With GlcN6P as a ligand, the native sensor glmS ribozyme is integrated at the 5′- of phosphoglucosamine mutase and 6-phosphofructokinase genes to decrease the flux dynamically toward the peptidoglycan synthesis and glycolysis pathway, respectively. The glmS ribozyme mutant M5 (glmS ribozyme cleavage site AG → GG) with decreased ribozyme activity is integrated at the 5′- of glucose-6-phosphate isomerase gene to increase the flux dynamically toward the GlcNAc synthesis pathway. This strategy increased the GlcNAc titer from 9.24 to 18.45 g/L, and the specific GlcNAc productivity from 0.53 to 1.21 g GlcNAc/g cell. Since GlcN6P is involved in the biosynthesis of various products, here the developed strategy for multiple target dynamic engineering of metabolic pathways can be generally used in B. subtilis and other industrial microbes for chemical production.
中文翻译:

工程化的氨基葡萄糖6磷酸盐反应性glmS核酶开关能够动态控制枯草芽孢杆菌中代谢通量的过量生产N-乙酰氨基葡萄糖
枯草芽孢杆菌是一种典型的工业微生物,广泛用于工业生物技术,特别是用于营养保健品生产。关于枯草芽孢杆菌的静态代谢工程的研究很多,而由于缺乏适当的元素,关于动态代谢工程的报道很少。在这里,我们建立了一种动态的重编程策略,用于重建枯草芽孢杆菌的代谢网络,使用典型的营养保健品N-乙酰氨基葡萄糖(GlcNAc)作为模型产品,而glmS(编码6氨基葡萄糖磷酸酶)的核酶是工程分子。 。首先,引入了trp终止子以有效释放glmS核酶反馈抑制。此外,我们设计了天然的6-磷酸氨基葡萄糖(GlcN6P)响应性glmS核酶开关,以动态控制枯草芽孢杆菌的代谢通量,以过量生产GlcNAc。以GlcN6P为配体,天然传感器glmS核酶整合在磷酸葡萄糖胺变位酶和6-磷酸果糖激酶基因的5'-处,以分别动态降低通向肽聚糖合成和糖酵解途径的通量。的GLMS核酶突变体M5(GLMS具有降低的核酶活性的核酶切割位点AG→GG)整合在6-磷酸葡萄糖异构酶基因的5'-上,以动态增加通向GlcNAc合成途径的通量。这种策略将GlcNAc滴度从9.24 g / L提高到18.45 g / L,比GlcNAc的生产率从0.53 g / l细胞提高到1.21 g / g细胞。由于GlcN6P参与了各种产品的生物合成,因此,针对代谢途径的多目标动态工程化开发的策略通常可用于枯草芽孢杆菌和其他工业微生物的化学生产。
更新日期:2018-08-23
中文翻译:

工程化的氨基葡萄糖6磷酸盐反应性glmS核酶开关能够动态控制枯草芽孢杆菌中代谢通量的过量生产N-乙酰氨基葡萄糖
枯草芽孢杆菌是一种典型的工业微生物,广泛用于工业生物技术,特别是用于营养保健品生产。关于枯草芽孢杆菌的静态代谢工程的研究很多,而由于缺乏适当的元素,关于动态代谢工程的报道很少。在这里,我们建立了一种动态的重编程策略,用于重建枯草芽孢杆菌的代谢网络,使用典型的营养保健品N-乙酰氨基葡萄糖(GlcNAc)作为模型产品,而glmS(编码6氨基葡萄糖磷酸酶)的核酶是工程分子。 。首先,引入了trp终止子以有效释放glmS核酶反馈抑制。此外,我们设计了天然的6-磷酸氨基葡萄糖(GlcN6P)响应性glmS核酶开关,以动态控制枯草芽孢杆菌的代谢通量,以过量生产GlcNAc。以GlcN6P为配体,天然传感器glmS核酶整合在磷酸葡萄糖胺变位酶和6-磷酸果糖激酶基因的5'-处,以分别动态降低通向肽聚糖合成和糖酵解途径的通量。的GLMS核酶突变体M5(GLMS具有降低的核酶活性的核酶切割位点AG→GG)整合在6-磷酸葡萄糖异构酶基因的5'-上,以动态增加通向GlcNAc合成途径的通量。这种策略将GlcNAc滴度从9.24 g / L提高到18.45 g / L,比GlcNAc的生产率从0.53 g / l细胞提高到1.21 g / g细胞。由于GlcN6P参与了各种产品的生物合成,因此,针对代谢途径的多目标动态工程化开发的策略通常可用于枯草芽孢杆菌和其他工业微生物的化学生产。



























 京公网安备 11010802027423号
京公网安备 11010802027423号