当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Applications of Radical Carbonylation and Amine Addition Chemistry: 1,4-Hydrogen Transfer of 1-Hydroxylallyl Radicals
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1021/acs.accounts.8b00278 Hiroshi Matsubara,Takuji Kawamoto,Takahide Fukuyama,Ilhyong Ryu
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1021/acs.accounts.8b00278 Hiroshi Matsubara,Takuji Kawamoto,Takahide Fukuyama,Ilhyong Ryu
|
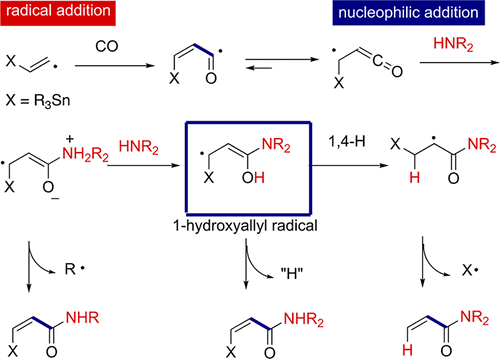
1,4-Hydrogen transfer from the 1-hydroxyallyl radical to give the enoxyl (α-keto) radical is an exothermic process with a high activation energy based on DFT calculations. The lack of experimental examples of such 1,4-H shift reactions lies in the difficulty of generating the 1-hydroxyallyl radical. We have shown that radical carbonylation of alkenyl radicals with CO followed by nucleophilic trapping of the carbonyl portion of the resulting radical by amines gives rise to 1-amino-substituted 1-hydroxyallyl radicals in situ. At the outset of this chemistry, we examined intramolecular trapping reactions via radical carbonylation of alkynylamines mediated by tributyltin hydride. Consequently, α-methylene lactams were obtained, for which the initially formed 1-amino-substituted 1-hydroxyallyl radical underwent a 1,4-H shift followed by subsequent β-scission, which led to the expulsion of a tributyltin radical. A competing pathway of the 1,4-H shift of 1-amino-substituted 1-hydroxyallyl radicals involving hydrogen abstraction was observed, which led to the formation of α-stannylmethylene lactams as a major byproduct. However, in contrast, when intermolecular trapping of α-ketenyl radicals by amines was carried out, the 1,4-H shift from the 1-amino-substituted 1-hydroxyallyl radical became the major pathway, which gave good yields of α,β-unsaturated amides. Thus, we were able to develop three-component reactions comprising terminal alkynes, CO, and amines that led to α,β-unsaturated amides via the 1,4-H shift reaction. DFT calculations support the observation that the 1,4-H shift is more facile when 1-hydroxyallyl radicals have both 1-amino and 3-tin substituents. The choice of substituents on the amine nitrogen is also important, since N–C bond cleavage via an SH2-type reaction can become a competing pathway. Such an unusual SH2-type reaction at the amine nitrogen is favored when the leaving alkyl radicals are stable, such as PhC(•)H(CH3) and t-Bu•. Interestingly, even nucleophilic attack of tertiary amines onto α-ketenyl radicals causes cleavage of the C–N bond. For this reaction, DFT calculations predict an indirect homolytic substitution mechanism involving expulsion of alkyl radicals through the zwitterionic radical intermediate arising from nucleophilic amine addition onto the α-ketenyl radical. In contrast, the carbonylation of aryl radicals, generated from aryl iodides, in the presence of amines gave aromatic carboxylic amides in good yields. It is proposed that radical anions originating from acyl radicals and amines undergo electron transfer to aryl iodides to give aminocarbonylation products.
中文翻译:

自由基羰基化和胺加成化学的应用:1-羟基烯丙基自由基的1,4-氢转移
从1-羟基烯丙基自由基进行1,4-氢转移以生成烯氧基(α-酮)自由基是一种放热过程,具有很高的活化能,基于DFT计算。缺乏这种1,4-H转移反应的实验例子在于难以产生1-羟基烯丙基。我们已经表明,用CO进行烯基自由基的自由基羰基化,然后通过胺的亲核捕集所得自由基的羰基部分会原位产生1-氨基取代的1-羟基烯丙基。在这种化学反应的开始,我们通过氢化三丁基锡介导的炔基胺的自由基羰基化研究了分子内捕获反应。结果,获得了α-亚甲基内酰胺,其最初形成的1-氨基取代的1-羟基烯丙基经历了1,发生4-H位移,随后发生β断裂,导致三丁基锡自由基被驱逐出境。观察到1-氨基取代的1-羟基烯丙基的1,4-H转移的竞争途径涉及夺氢,这导致形成α-锡烷基亚甲基内酰胺为主要副产物。然而,相比之下,当通过胺分子间捕获α-烯基自由基时,从1-氨基取代的1-羟基烯丙基的1,4-H转变成为主要途径,这提供了良好的α,β收率。 -不饱和酰胺。因此,我们能够开发出三组分反应,包括末端炔烃,CO和胺,它们通过1,4-H转变反应生成α,β-不饱和酰胺。DFT计算支持以下观察:当1-羟基烯丙基同时具有1-氨基和3-锡取代基时,4-H移位更容易。胺氮上取代基的选择也很重要,因为N–C键可通过S裂解H 2型反应可以成为竞争途径。当剩下的烷基如PhC(•)H(CH 3)和t稳定时,在胺氮上发生这种异常的S H 2型反应是有利的。-Bu•。有趣的是,即使是叔胺对α-烯基自由基的亲核攻击也会导致C–N键的断裂。对于该反应,DFT计算预测了一种间接均质取代机制,该机制涉及通过将亲核胺加成到α-烯基上而产生的两性离子自由基中间体将烷基自由基驱逐出去。相反,在胺存在下由芳基碘化物产生的芳基的羰基化以良好的收率得到芳族羧酸酰胺。提出源自酰基和胺的自由基阴离子经历电子转移至芳基碘化物以产生氨基羰基化产物。
更新日期:2018-08-23
中文翻译:

自由基羰基化和胺加成化学的应用:1-羟基烯丙基自由基的1,4-氢转移
从1-羟基烯丙基自由基进行1,4-氢转移以生成烯氧基(α-酮)自由基是一种放热过程,具有很高的活化能,基于DFT计算。缺乏这种1,4-H转移反应的实验例子在于难以产生1-羟基烯丙基。我们已经表明,用CO进行烯基自由基的自由基羰基化,然后通过胺的亲核捕集所得自由基的羰基部分会原位产生1-氨基取代的1-羟基烯丙基。在这种化学反应的开始,我们通过氢化三丁基锡介导的炔基胺的自由基羰基化研究了分子内捕获反应。结果,获得了α-亚甲基内酰胺,其最初形成的1-氨基取代的1-羟基烯丙基经历了1,发生4-H位移,随后发生β断裂,导致三丁基锡自由基被驱逐出境。观察到1-氨基取代的1-羟基烯丙基的1,4-H转移的竞争途径涉及夺氢,这导致形成α-锡烷基亚甲基内酰胺为主要副产物。然而,相比之下,当通过胺分子间捕获α-烯基自由基时,从1-氨基取代的1-羟基烯丙基的1,4-H转变成为主要途径,这提供了良好的α,β收率。 -不饱和酰胺。因此,我们能够开发出三组分反应,包括末端炔烃,CO和胺,它们通过1,4-H转变反应生成α,β-不饱和酰胺。DFT计算支持以下观察:当1-羟基烯丙基同时具有1-氨基和3-锡取代基时,4-H移位更容易。胺氮上取代基的选择也很重要,因为N–C键可通过S裂解H 2型反应可以成为竞争途径。当剩下的烷基如PhC(•)H(CH 3)和t稳定时,在胺氮上发生这种异常的S H 2型反应是有利的。-Bu•。有趣的是,即使是叔胺对α-烯基自由基的亲核攻击也会导致C–N键的断裂。对于该反应,DFT计算预测了一种间接均质取代机制,该机制涉及通过将亲核胺加成到α-烯基上而产生的两性离子自由基中间体将烷基自由基驱逐出去。相反,在胺存在下由芳基碘化物产生的芳基的羰基化以良好的收率得到芳族羧酸酰胺。提出源自酰基和胺的自由基阴离子经历电子转移至芳基碘化物以产生氨基羰基化产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号