当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quantitative Live-Cell Kinetic Degradation and Mechanistic Profiling of PROTAC Mode of Action
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1021/acschembio.8b00692 Kristin M. Riching 1 , Sarah Mahan 1 , Cesear R. Corona 2 , Mark McDougall 2 , James D. Vasta 1 , Matthew B. Robers 1 , Marjeta Urh 1 , Danette L. Daniels 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1021/acschembio.8b00692 Kristin M. Riching 1 , Sarah Mahan 1 , Cesear R. Corona 2 , Mark McDougall 2 , James D. Vasta 1 , Matthew B. Robers 1 , Marjeta Urh 1 , Danette L. Daniels 1
Affiliation
|
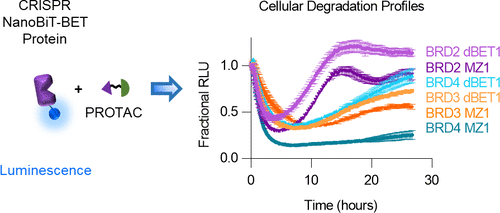
A new generation of heterobifunctional small molecules, termed proteolysis targeting chimeras (PROTACs), targets proteins for degradation through recruitment to E3 ligases and holds significant therapeutic potential. Despite numerous successful examples, PROTAC small molecule development remains laborious and unpredictable, involving testing compounds for end-point degradation activity at fixed times and concentrations without resolving or optimizing for the important biological steps required for the process. Given the complexity of the ubiquitin proteasomal pathway, technologies that enable real-time characterization of PROTAC efficacy and mechanism of action are critical for accelerating compound development, profiling, and improving guidance of chemical structure–activity relationship. Here, we present an innovative, modular live-cell platform utilizing endogenous tagging technologies and apply it to monitoring PROTAC-mediated degradation of the bromodomain and extra-terminal family members. We show comprehensive real-time degradation and recovery profiles for each target, precisely quantifying degradation rates, maximal levels of degradation (Dmax), and time frame at Dmax. These degradation metrics show specific PROTAC and family member-dependent responses that are closely associated with the key cellular protein interactions required for the process. Kinetic studies show cellular ternary complex stability influences potency and degradation efficacy. Meanwhile, the level of ubiquitination is highly correlated to degradation rate, indicating ubiquitination stemming from productive ternary complex formation is the main driver of the degradation rate. The approaches applied here highlight the steps at which the choice of E3 ligase handle can elicit different outcomes and discern individual parameters required for degradation, ultimately enabling chemical design strategies and rank ordering of potential therapeutic compounds.
中文翻译:

PROTAC作用方式的定量活细胞动力学降解和机理分析
新一代异双功能小分子,称为针对嵌合体(PROTAC)的蛋白水解,可通过募集E3连接酶来靶向蛋白质降解,并具有巨大的治疗潜力。尽管有许多成功的例子,但PROTAC小分子的开发仍然费力且不可预测,涉及在固定的时间和浓度下测试化合物的端点降解活性,而没有解决或优化该过程所需的重要生物学步骤。考虑到泛素蛋白酶体途径的复杂性,能够实时表征PROTAC功效和作用机制的技术对于加速化合物的开发,分析和改善化学结构-活性关系的指导至关重要。在这里,我们提出一种创新的方法,模块化活细胞平台利用内源性标记技术,并将其应用于监测PROTAC介导的溴结构域和末端外家族成员的降解。我们为每个目标显示了全面的实时降解和恢复曲线,精确量化了降解速率,最大降解水平(D max),以及D max的时间范围。这些降解指标显示出特定的PROTAC和依赖家族成员的反应,这些反应与该过程所需的关键细胞蛋白质相互作用密切相关。动力学研究表明细胞三元复合物的稳定性影响效能和降解功效。同时,泛素化水平与降解速率高度相关,表明源自生产性三元复合物形成的泛素化是降解速率的主要驱动力。此处应用的方法重点介绍了选择E3连接酶处理可引发不同结果并识别降解所需的各个参数的步骤,从而最终实现了化学设计策略和潜在治疗化合物的排序。
更新日期:2018-08-23
中文翻译:

PROTAC作用方式的定量活细胞动力学降解和机理分析
新一代异双功能小分子,称为针对嵌合体(PROTAC)的蛋白水解,可通过募集E3连接酶来靶向蛋白质降解,并具有巨大的治疗潜力。尽管有许多成功的例子,但PROTAC小分子的开发仍然费力且不可预测,涉及在固定的时间和浓度下测试化合物的端点降解活性,而没有解决或优化该过程所需的重要生物学步骤。考虑到泛素蛋白酶体途径的复杂性,能够实时表征PROTAC功效和作用机制的技术对于加速化合物的开发,分析和改善化学结构-活性关系的指导至关重要。在这里,我们提出一种创新的方法,模块化活细胞平台利用内源性标记技术,并将其应用于监测PROTAC介导的溴结构域和末端外家族成员的降解。我们为每个目标显示了全面的实时降解和恢复曲线,精确量化了降解速率,最大降解水平(D max),以及D max的时间范围。这些降解指标显示出特定的PROTAC和依赖家族成员的反应,这些反应与该过程所需的关键细胞蛋白质相互作用密切相关。动力学研究表明细胞三元复合物的稳定性影响效能和降解功效。同时,泛素化水平与降解速率高度相关,表明源自生产性三元复合物形成的泛素化是降解速率的主要驱动力。此处应用的方法重点介绍了选择E3连接酶处理可引发不同结果并识别降解所需的各个参数的步骤,从而最终实现了化学设计策略和潜在治疗化合物的排序。



























 京公网安备 11010802027423号
京公网安备 11010802027423号