当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The mechanism of the triple aryne–tetrazine reaction cascade: theory and experiment†
Chemical Science ( IF 8.4 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1039/c8sc01796d Sung-Eun Suh 1 , Shuming Chen 2 , K N Houk 2 , David M Chenoweth 1
Chemical Science ( IF 8.4 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1039/c8sc01796d Sung-Eun Suh 1 , Shuming Chen 2 , K N Houk 2 , David M Chenoweth 1
Affiliation
|
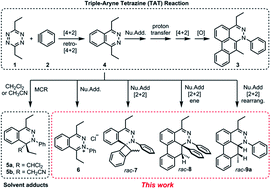
This article describes an experimental and computational investigation on the possible aryne reactivity modes in the course of the reaction of two highly energetic molecules, an aryne and a 1,2,4,5-tetrazine. Beyond the triple aryne–tetrazine (TAT) reaction, it was observed that combinations of several reactivity modes afford several heterocyclic compounds. Density Functional Theory (DFT) calculations of competition between a second Diels–Alder reaction and the nucleophilic addition pathways indicates the latter to be more favorable. Crossover experiments and computational study of the proton transfer step reveal that the reaction proceeds intermolecularly with the assistance of a water molecule, rather than intramolecularly. The resulting enamine intermediate was found to undergo either a stepwise formal [2 + 2] or [4 + 2] cycloaddition, and their energetic profiles were compared against each other. Isolation of an ene-product and a rearranged product shows the potential competition with oxidation/desaturation. These studies show how multiple arynes react with a highly reactive starting material and provide guidance for future applications of aryne-based multicomponent cascade reactions.
中文翻译:

三芳炔-四嗪级联反应的机理:理论与实验†
本文描述了对芳炔和 1,2,4,5-四嗪这两种高能分子反应过程中可能的芳烃反应模式的实验和计算研究。除了三芳基-四嗪 (TAT) 反应之外,观察到几种反应模式的组合提供了几种杂环化合物。第二个 Diels-Alder 反应和亲核加成途径之间竞争的密度泛函理论 (DFT) 计算表明后者更有利。质子转移步骤的交叉实验和计算研究表明,反应在水分子的帮助下在分子间进行,而不是在分子内进行。发现所得烯胺中间体经历了逐步形式的 [2 + 2] 或 [4 + 2] 环加成反应,并将他们的精力充沛的资料相互比较。烯产物和重排产物的分离显示了与氧化/去饱和的潜在竞争。这些研究显示了多种芳烃如何与高活性起始材料发生反应,并为基于芳烃的多组分级联反应的未来应用提供指导。
更新日期:2018-08-23
中文翻译:

三芳炔-四嗪级联反应的机理:理论与实验†
本文描述了对芳炔和 1,2,4,5-四嗪这两种高能分子反应过程中可能的芳烃反应模式的实验和计算研究。除了三芳基-四嗪 (TAT) 反应之外,观察到几种反应模式的组合提供了几种杂环化合物。第二个 Diels-Alder 反应和亲核加成途径之间竞争的密度泛函理论 (DFT) 计算表明后者更有利。质子转移步骤的交叉实验和计算研究表明,反应在水分子的帮助下在分子间进行,而不是在分子内进行。发现所得烯胺中间体经历了逐步形式的 [2 + 2] 或 [4 + 2] 环加成反应,并将他们的精力充沛的资料相互比较。烯产物和重排产物的分离显示了与氧化/去饱和的潜在竞争。这些研究显示了多种芳烃如何与高活性起始材料发生反应,并为基于芳烃的多组分级联反应的未来应用提供指导。


























 京公网安备 11010802027423号
京公网安备 11010802027423号