当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Biobased Polyester Polyol through Esterification of Sorbitol with Azelaic Acid Catalyzed by Tin(II) Oxide: A Kinetic Modeling Study
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2018-09-04 , DOI: 10.1021/acs.iecr.8b02506 M. R. Kamaruzaman 1 , S. Y. Chin 1, 2 , E. C. L. Pui 1 , H. Prasetiawan 3 , Nurwadiah Azizan 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2018-09-04 , DOI: 10.1021/acs.iecr.8b02506 M. R. Kamaruzaman 1 , S. Y. Chin 1, 2 , E. C. L. Pui 1 , H. Prasetiawan 3 , Nurwadiah Azizan 1
Affiliation
|
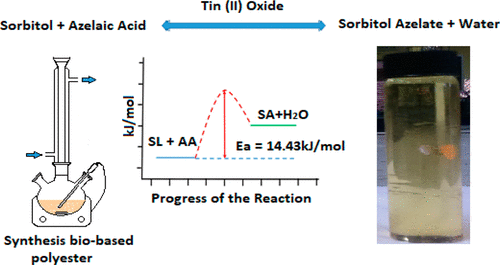
A sustainable and renewable biobased polyester polyol for polyurethane production was synthesized through the esterification of azelaic acid and sorbitol catalyzed by tin(II) oxide in a batch system. The studies on chemical equilibrium, reaction kinetics and important operating parameters were carried out. The temperature, molar ratio of sorbitol to azelaic acid and catalyst loading were varied in order to determine the best reaction conditions. The polyester polyol synthesized was tested for its fatty acid content through titration. The best operating condition found was at reaction temperature of 433 K, sorbitol to azelaic acid molar ratio of 4:1 and catalyst loading of 2 wt %, yielding 72% azelaic acid conversion after 6 h of reaction. The presence of minute amount of sorbitan and isosorbide inferred the potential of sorbitol-based branched polyester formation with its backbone incorporated with these sorbitol anhydrides. The equilibrium study validated the endothermicity of the reaction. Meanwhile, the kinetic data well fitted to the Langmuir–Hinshelwood Hougen Watson (LHHW) model with the activation energy of 14.43 kJ/mol.
中文翻译:

氧化锡(II)催化壬二酸山梨糖醇酯化合成生物基聚酯多元醇的动力学模型研究
通过将壬二酸和山梨糖醇在氧化锡(II)的催化下分批系统进行酯化反应,合成了一种可持续的,可再生的生物基聚氨酯多元醇,用于聚氨酯生产。对化学平衡,反应动力学和重要的操作参数进行了研究。为了确定最佳反应条件,改变温度,山梨糖醇与壬二酸的摩尔比和催化剂的负载量。通过滴定测试合成的聚酯多元醇的脂肪酸含量。发现的最佳操作条件是反应温度为433 K,山梨糖醇与壬二酸的摩尔比为4:1,催化剂负载量为2 wt%,反应6小时后,壬二酸转化率为72%。微量的失水山梨糖醇和异山梨醇的存在暗示了其骨架与这些山梨糖醇酸酐结合的基于山梨糖醇基的支化聚酯的形成潜力。平衡研究验证了反应的吸热性。同时,动力学数据非常适合Langmuir-Hinshelwood Hougen Watson(LHHW)模型,其活化能为14.43 kJ / mol。
更新日期:2018-09-05
中文翻译:

氧化锡(II)催化壬二酸山梨糖醇酯化合成生物基聚酯多元醇的动力学模型研究
通过将壬二酸和山梨糖醇在氧化锡(II)的催化下分批系统进行酯化反应,合成了一种可持续的,可再生的生物基聚氨酯多元醇,用于聚氨酯生产。对化学平衡,反应动力学和重要的操作参数进行了研究。为了确定最佳反应条件,改变温度,山梨糖醇与壬二酸的摩尔比和催化剂的负载量。通过滴定测试合成的聚酯多元醇的脂肪酸含量。发现的最佳操作条件是反应温度为433 K,山梨糖醇与壬二酸的摩尔比为4:1,催化剂负载量为2 wt%,反应6小时后,壬二酸转化率为72%。微量的失水山梨糖醇和异山梨醇的存在暗示了其骨架与这些山梨糖醇酸酐结合的基于山梨糖醇基的支化聚酯的形成潜力。平衡研究验证了反应的吸热性。同时,动力学数据非常适合Langmuir-Hinshelwood Hougen Watson(LHHW)模型,其活化能为14.43 kJ / mol。



























 京公网安备 11010802027423号
京公网安备 11010802027423号