当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Impact of Post-CCSD(T) Corrections on Reaction Energetics and Rate Constants of the OH• + HCl Reaction
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-08-20 00:00:00 , DOI: 10.1021/acs.jpca.8b06092 Subhasish Mallick 1 , Pradeep Kumar 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-08-20 00:00:00 , DOI: 10.1021/acs.jpca.8b06092 Subhasish Mallick 1 , Pradeep Kumar 1
Affiliation
|
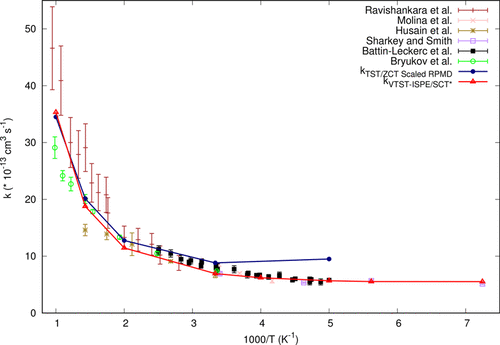
High level ab initio calculations have been performed to predict the reaction energy and barrier height for the OH• + HCl reaction. After including the effect of full quadratic excitations at the coupled cluster level, in addition to core, relativistic, spin–orbit, and diagonal Born–Oppenheimer corrections, we found the values of reaction energy and barrier height to be −15.29 and +2.38 kcal mol–1, respectively. Employing this reaction energy and barrier height, we used variational transition state theory in conjunction with small curvature tunneling to calculate the rate constants within a temperature range from 138 to 1000 K. The calculated rate constants were found to be in good agreement with available experimental results throughout the whole temperature range.
中文翻译:

后CCSD(T)校正对OH • + HCl反应的反应能和速率常数的影响
已经进行了高水平的从头计算,以预测OH • + HCl反应的反应能量和势垒高度。在包括耦合簇级的完全二次激发的影响之后,除了核心,相对论,自旋轨道和对角的Born-Oppenheimer校正之外,我们发现反应能和势垒高度的值分别为−15.29和+2.38 kcal mol –1分别。利用这种反应能和势垒高度,我们结合变小过渡态理论和小曲率隧穿来计算138至1000 K温度范围内的速率常数。发现该速率常数与可用的实验结果非常吻合。在整个温度范围内。
更新日期:2018-08-20
中文翻译:

后CCSD(T)校正对OH • + HCl反应的反应能和速率常数的影响
已经进行了高水平的从头计算,以预测OH • + HCl反应的反应能量和势垒高度。在包括耦合簇级的完全二次激发的影响之后,除了核心,相对论,自旋轨道和对角的Born-Oppenheimer校正之外,我们发现反应能和势垒高度的值分别为−15.29和+2.38 kcal mol –1分别。利用这种反应能和势垒高度,我们结合变小过渡态理论和小曲率隧穿来计算138至1000 K温度范围内的速率常数。发现该速率常数与可用的实验结果非常吻合。在整个温度范围内。


























 京公网安备 11010802027423号
京公网安备 11010802027423号