当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modulation of the Aggregation of the Prion-like Protein RepA-WH1 by Chaperones in a Cell-Free Expression System and in Cytomimetic Lipid Vesicles
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2018-08-20 00:00:00 , DOI: 10.1021/acssynbio.8b00283 Cristina Fernández 1 , Rafael Giraldo 1
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2018-08-20 00:00:00 , DOI: 10.1021/acssynbio.8b00283 Cristina Fernández 1 , Rafael Giraldo 1
Affiliation
|
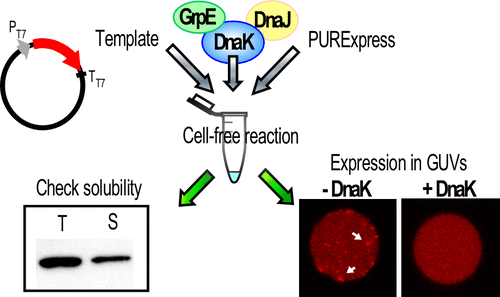
The accumulation of aggregated forms of proteins as toxic species is associated with fatal diseases such as amyloid proteinopathies. With the purpose of deconstructing the molecular mechanisms of these type of diseases through a Synthetic Biology approach, we are working with a model bacterial prion-like protein, RepA-WH1, expressed in a cell-free system. Our findings show that the Hsp70 chaperone from Escherichia coli, together with its Hsp40 and nucleotide exchange factor cochaperones, modulates the aggregation of the prion-like protein in the cell-free system. Moreover, we observe the same effect by reconstructing the aggregation process inside lipid vesicles. Chaperones reduce the number of aggregates formed, matching previous findings in vivo. We expect that the in vitro approach reported here will help to achieve better understanding and control of amyloid proteinopathies.
中文翻译:

伴侣在无细胞表达系统和拟液化脂质囊泡中的伴侣蛋白对on病毒样蛋白RepA-WH1聚集的调节。
聚集形式的蛋白质作为有毒物质的积累与致命疾病如淀粉样蛋白病有关。为了通过合成生物学方法解构这些类型疾病的分子机制,我们正在研究在无细胞系统中表达的模型细菌ion病毒样蛋白RepA-WH1。我们的发现表明,来自大肠杆菌的Hsp70分子伴侣,连同其Hsp40和核苷酸交换因子伴侣分子,可调节无细胞系统中pr病毒样蛋白的聚集。此外,我们通过重建脂质囊泡内部的聚集过程观察到相同的效果。分子伴侣减少了形成的聚集体的数量,与体内先前的发现相符。我们希望本文报道的体外方法将有助于更好地了解和控制淀粉样蛋白病。
更新日期:2018-08-20
中文翻译:

伴侣在无细胞表达系统和拟液化脂质囊泡中的伴侣蛋白对on病毒样蛋白RepA-WH1聚集的调节。
聚集形式的蛋白质作为有毒物质的积累与致命疾病如淀粉样蛋白病有关。为了通过合成生物学方法解构这些类型疾病的分子机制,我们正在研究在无细胞系统中表达的模型细菌ion病毒样蛋白RepA-WH1。我们的发现表明,来自大肠杆菌的Hsp70分子伴侣,连同其Hsp40和核苷酸交换因子伴侣分子,可调节无细胞系统中pr病毒样蛋白的聚集。此外,我们通过重建脂质囊泡内部的聚集过程观察到相同的效果。分子伴侣减少了形成的聚集体的数量,与体内先前的发现相符。我们希望本文报道的体外方法将有助于更好地了解和控制淀粉样蛋白病。


























 京公网安备 11010802027423号
京公网安备 11010802027423号