Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Microfluidic auto-alignment of protein patterns for dissecting multi-receptor crosstalk in platelets
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-08-20 00:00:00 , DOI: 10.1039/c8lc00464a F Zhou 1 , Y Chen , E I Felner , C Zhu , H Lu
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-08-20 00:00:00 , DOI: 10.1039/c8lc00464a F Zhou 1 , Y Chen , E I Felner , C Zhu , H Lu
Affiliation
|
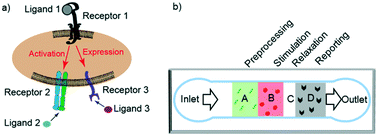
Cell adhesion plays a critical role in many cellular functions, such as the hemostatic/thrombotic process, inflammatory reactions, and adaptive immune response. Many cell adhesion processes involve crosstalk between multiple ligand–receptor systems through intracellular signaling. To elucidate such crosstalk requires analysis of the synergistic or antagonistic effects of binding and signalling of multi-receptor species. Current techniques for these analyses, e.g., atomic force microscopy (AFM) and biomembrane force probe (BFP) assays, are either labor-intensive, low-throughput, or limited in the types of ligands they can interrogate. Circumventing these limitations requires a technique for manipulating ligand interactions with and measuring the functional response of a population of cells. In this work, we have developed a microfluidic platform for studying the binding and signaling of multi-receptor species by separating their actions in space and time. The platform directs cells through a single channel and uses sequentially presented ligands for pre-processing and stimulating cells, followed by reporting of cell activation states and functional consequences. Our method precisely patterns multiple proteins in different spatial regions without gaps. We demonstrate the utility of our method by using this platform to analyze the crosstalk between platelet receptors, glycoprotein Ib and IIb–IIIa, in the context of platelet adhesion and signaling under flow. We show the clinical utility of this platform by applying it to analyze whole blood samples and to assess differences in the activation of platelets between healthy and diabetic patients.
中文翻译:

蛋白质模式的微流体自动对齐用于解剖血小板中的多受体串扰
细胞粘附在许多细胞功能中发挥着关键作用,例如止血/血栓形成过程、炎症反应和适应性免疫反应。许多细胞粘附过程涉及多个配体-受体系统之间通过细胞内信号传导的串扰。为了阐明这种串扰,需要分析多受体种类的结合和信号传导的协同或拮抗作用。目前用于这些分析的技术,例如原子力显微镜(AFM)和生物膜力探针(BFP)测定,要么是劳动密集型的、低通量的,要么是它们可以询问的配体类型有限。规避这些限制需要一种操纵配体与细胞群相互作用并测量细胞群功能反应的技术。在这项工作中,我们开发了一个微流体平台,通过分离空间和时间上的作用来研究多受体物种的结合和信号传导。该平台通过单一通道引导细胞,并使用顺序呈现的配体来预处理和刺激细胞,然后报告细胞激活状态和功能结果。我们的方法精确地对不同空间区域中的多种蛋白质进行无间隙的图案化。我们通过使用该平台分析血小板受体、糖蛋白 Ib 和 IIb-IIIa 之间在血小板粘附和流动下信号传导的背景下的串扰来证明我们方法的实用性。我们通过应用该平台分析全血样本并评估健康患者和糖尿病患者之间血小板活化的差异来展示该平台的临床实用性。
更新日期:2018-08-20
中文翻译:

蛋白质模式的微流体自动对齐用于解剖血小板中的多受体串扰
细胞粘附在许多细胞功能中发挥着关键作用,例如止血/血栓形成过程、炎症反应和适应性免疫反应。许多细胞粘附过程涉及多个配体-受体系统之间通过细胞内信号传导的串扰。为了阐明这种串扰,需要分析多受体种类的结合和信号传导的协同或拮抗作用。目前用于这些分析的技术,例如原子力显微镜(AFM)和生物膜力探针(BFP)测定,要么是劳动密集型的、低通量的,要么是它们可以询问的配体类型有限。规避这些限制需要一种操纵配体与细胞群相互作用并测量细胞群功能反应的技术。在这项工作中,我们开发了一个微流体平台,通过分离空间和时间上的作用来研究多受体物种的结合和信号传导。该平台通过单一通道引导细胞,并使用顺序呈现的配体来预处理和刺激细胞,然后报告细胞激活状态和功能结果。我们的方法精确地对不同空间区域中的多种蛋白质进行无间隙的图案化。我们通过使用该平台分析血小板受体、糖蛋白 Ib 和 IIb-IIIa 之间在血小板粘附和流动下信号传导的背景下的串扰来证明我们方法的实用性。我们通过应用该平台分析全血样本并评估健康患者和糖尿病患者之间血小板活化的差异来展示该平台的临床实用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号