当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solvent Effects on the Reversible Intercalation of Magnesium‐Ions into V2O5 Electrodes
ChemElectroChem ( IF 4 ) Pub Date : 2018-09-05 , DOI: 10.1002/celc.201800932 Ran Attias 1 , Michael Salama 1 , Baruch Hirsch 1 , Yosef Gofer 1 , Doron Aurbach 1
ChemElectroChem ( IF 4 ) Pub Date : 2018-09-05 , DOI: 10.1002/celc.201800932 Ran Attias 1 , Michael Salama 1 , Baruch Hirsch 1 , Yosef Gofer 1 , Doron Aurbach 1
Affiliation
|
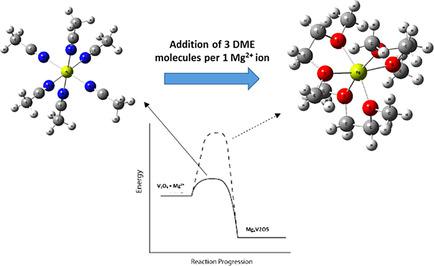
The choice for solvents in electrolyte solutions for non‐aqueous magnesium batteries is currently limited to ethers. However, the scientific community regularly uses acetonitrile (ACN) based electrolyte solutions as model systems in order to characterize new cathode materials for rechargeable Magnesium batteries. In this study, we demonstrated the effect of dimethoxyethane (DME), an important solvent for rechargeable magnesium systems, on the reversible intercalation of Mg2+ cations into thin, monolithic V2O5 films. The effect of DME on the intercalation kinetic have been examined via chronopotentiometry, galvanostatic and galvanostatic intermittent titration (GITT) measurements. In addition, we explored basic scientific questions related to the structure of ACN:DME based electrolyte solutions via Raman spectroscopy and surface chemistry of V2O5/Mg(ClO4)2/ACN:DME systems via X‐ray photoelectron spectroscopy. We found that addition of DME to the Mg(ClO4)2/ACN solution results in replacing the ACN‐Mg2+ cation by the more thermodynamically stable 3DME‐Mg2+ solvate structure. We also found that the DME‐Mg interaction forms stable solution structures that kinetically slow down the insertion of the Mg2+ cations into V2O5, compared with the less coordinated situation in solutions based solely on ACN.
中文翻译:

溶剂对镁离子可逆嵌入V2O5电极的影响
当前,非水镁电池电解液中溶剂的选择仅限于醚。但是,科学界经常使用基于乙腈(ACN)的电解质溶液作为模型系统,以表征可充电镁电池的新阴极材料。在这项研究中,我们证明了二甲氧基乙烷(DME)(一种可再充电镁系统的重要溶剂)对Mg 2+阳离子可逆插入到薄的整体V 2 O 5中的作用。电影。DME对插层动力学的影响已通过计时电位法,恒电流和恒电流间歇滴定(GITT)测量进行了检查。此外,我们通过拉曼光谱探索了与基于ACN:DME的电解质溶液的结构有关的基本科学问题,并通过X射线光电子能谱研究了V 2 O 5 / Mg(ClO 4)2 / ACN:DME系统的表面化学。我们发现,在Mg(ClO 4)2 / ACN溶液中添加二甲醚会导致热力学更稳定的3DME-Mg 2+取代ACN-Mg 2+阳离子。溶剂化物结构。我们还发现,与仅基于ACN的溶液中协调性较差的情况相比,DME-Mg相互作用形成了稳定的溶液结构,该结构在动力学上减慢了Mg 2+阳离子向V 2 O 5的插入。
更新日期:2018-09-05
中文翻译:

溶剂对镁离子可逆嵌入V2O5电极的影响
当前,非水镁电池电解液中溶剂的选择仅限于醚。但是,科学界经常使用基于乙腈(ACN)的电解质溶液作为模型系统,以表征可充电镁电池的新阴极材料。在这项研究中,我们证明了二甲氧基乙烷(DME)(一种可再充电镁系统的重要溶剂)对Mg 2+阳离子可逆插入到薄的整体V 2 O 5中的作用。电影。DME对插层动力学的影响已通过计时电位法,恒电流和恒电流间歇滴定(GITT)测量进行了检查。此外,我们通过拉曼光谱探索了与基于ACN:DME的电解质溶液的结构有关的基本科学问题,并通过X射线光电子能谱研究了V 2 O 5 / Mg(ClO 4)2 / ACN:DME系统的表面化学。我们发现,在Mg(ClO 4)2 / ACN溶液中添加二甲醚会导致热力学更稳定的3DME-Mg 2+取代ACN-Mg 2+阳离子。溶剂化物结构。我们还发现,与仅基于ACN的溶液中协调性较差的情况相比,DME-Mg相互作用形成了稳定的溶液结构,该结构在动力学上减慢了Mg 2+阳离子向V 2 O 5的插入。



























 京公网安备 11010802027423号
京公网安备 11010802027423号