当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Editing N-Glycan Site Occupancy with Small-Molecule Oligosaccharyltransferase Inhibitors
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2018-08-02 , DOI: 10.1016/j.chembiol.2018.07.005 Natalia Rinis , Jennifer E. Golden , Caleb D. Marceau , Jan E. Carette , Michael C. Van Zandt , Reid Gilmore , Joseph N. Contessa
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2018-08-02 , DOI: 10.1016/j.chembiol.2018.07.005 Natalia Rinis , Jennifer E. Golden , Caleb D. Marceau , Jan E. Carette , Michael C. Van Zandt , Reid Gilmore , Joseph N. Contessa
|
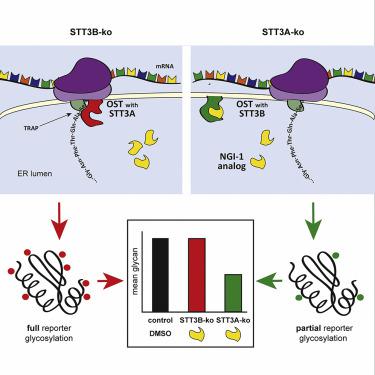
The oligosaccharyltransferase (OST) is a multisubunit enzyme complex that N-glycosylates proteins in the secretory pathway and is considered to be constitutive and unregulated. However, small-molecule OST inhibitors such as NGI-1 provide a pharmacological approach for regulating N-linked glycosylation. Herein we design cell models with knockout of each OST catalytic subunit (STT3A or STT3B) to screen the activity of NGI-1 and its analogs. We show that NGI-1 targets the function of both STT3A and STT3B and use structure-activity relationships to guide synthesis of catalytic subunit-specific inhibitors. Using this approach, pharmacophores that increase STT3B selectivity are characterized and an STT3B-specific inhibitor is identified. This inhibitor has discrete biological effects on endogenous STT3B target proteins such as COX2 but does not activate the cellular unfolded protein response. Together this work demonstrates that subsets of glycoproteins can be regulated through pharmacologic inhibition of N-linked glycosylation.
中文翻译:

使用小分子寡糖基转移酶抑制剂编辑N-聚糖位点占有率
寡糖基转移酶(OST)是一种多亚基酶复合物,其N-糖基化分泌途径中的蛋白质,被认为是组成性且不受调节的。但是,小分子OST抑制剂(例如NGI-1)为调节N-联糖基化提供了药理学方法。在本文中,我们设计了具有每个OST催化亚基(STT3A或STT3B)敲除的细胞模型,以筛选NGI-1及其类似物的活性。我们显示NGI-1既针对STT3A也针对STT3B的功能,并使用结构活性关系来指导催化亚基特异性抑制剂的合成。使用这种方法,表征增加STT3B选择性的药效团并鉴定出STT3B特异性抑制剂。该抑制剂对内源性STT3B靶蛋白(例如COX2)具有离散的生物学作用,但不会激活细胞未折叠的蛋白反应。这项工作在一起表明,糖蛋白的子集可以通过N联糖基化的药理抑制来调节。
更新日期:2018-10-19
中文翻译:

使用小分子寡糖基转移酶抑制剂编辑N-聚糖位点占有率
寡糖基转移酶(OST)是一种多亚基酶复合物,其N-糖基化分泌途径中的蛋白质,被认为是组成性且不受调节的。但是,小分子OST抑制剂(例如NGI-1)为调节N-联糖基化提供了药理学方法。在本文中,我们设计了具有每个OST催化亚基(STT3A或STT3B)敲除的细胞模型,以筛选NGI-1及其类似物的活性。我们显示NGI-1既针对STT3A也针对STT3B的功能,并使用结构活性关系来指导催化亚基特异性抑制剂的合成。使用这种方法,表征增加STT3B选择性的药效团并鉴定出STT3B特异性抑制剂。该抑制剂对内源性STT3B靶蛋白(例如COX2)具有离散的生物学作用,但不会激活细胞未折叠的蛋白反应。这项工作在一起表明,糖蛋白的子集可以通过N联糖基化的药理抑制来调节。



























 京公网安备 11010802027423号
京公网安备 11010802027423号