当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comprehensive Insight into the Hydrogen Bonding of Silanes
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2018-09-21 , DOI: 10.1002/asia.201801156 Evgenia D. Voronova 1 , Igor E. Golub 1, 2 , Alexander A. Pavlov 1 , Natalia V. Belkova 1 , Oleg A. Filippov 1 , Lina M. Epstein 1 , Elena S. Shubina 1
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2018-09-21 , DOI: 10.1002/asia.201801156 Evgenia D. Voronova 1 , Igor E. Golub 1, 2 , Alexander A. Pavlov 1 , Natalia V. Belkova 1 , Oleg A. Filippov 1 , Lina M. Epstein 1 , Elena S. Shubina 1
Affiliation
|
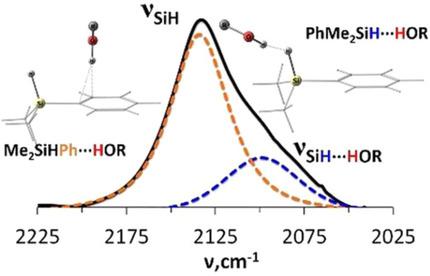
The interaction of a set of mono‐, di‐ and trisubstituted silanes with OH proton donors of different strength was studied by variable temperature (VT) FTIR and NMR spectroscopies at 190–298 K. Two competing sites of proton donors coordination: SiH and π‐density of phenyl rings—are revealed for phenyl‐containing silanes. The hydrogen bonds SiH⋅⋅⋅HO and OH⋅⋅⋅π(Ph) are of similar strength, but can be distinguished in the νSiH range: the νSiH⋅⋅⋅HO vibrations appear at lower frequencies while OH⋅⋅⋅π(Ph) complexes give Si–H vibrations shifted to higher frequency. The calculations showed the manifold picture of the noncovalent interactions in hydrogen‐bonded complexes of phenylsilanes. As OH⋅⋅⋅HSi bonds are weak, the other noncovalent interactions compete in the stabilization of the intermolecular complexes. Still, the structural and electronic parameters of “pure” DHB complexes of phenylsilanes are similar to those of Et3SiH.
中文翻译:

硅烷氢键的综合见解
通过在190–298 K的可变温度(VT)FTIR和NMR光谱研究了一组单取代,二取代和三取代的硅烷与不同强度的OH质子供体的相互作用。质子供体的两个竞争位点:SiH和π苯环的密度–显示了含苯基的硅烷。氢键SiH⋅⋅⋅HO和OH⋅⋅⋅π(PH)是相似的强度,但可以在ν来区分的SiH范围:在ν SiH⋅⋅⋅HO振动出现在较低频率,而OH⋅⋅⋅π(Ph)配合物使Si–H振动移至较高频率。计算结果显示了苯硅烷氢键键合配合物中非共价相互作用的多面性。由于OH⋅⋅⋅HSi键较弱,其他非共价相互作用在分子间复合物的稳定化中竞争。尽管如此,苯基硅烷的“纯” DHB配合物的结构和电子参数与Et 3 SiH相似。
更新日期:2018-09-21
中文翻译:

硅烷氢键的综合见解
通过在190–298 K的可变温度(VT)FTIR和NMR光谱研究了一组单取代,二取代和三取代的硅烷与不同强度的OH质子供体的相互作用。质子供体的两个竞争位点:SiH和π苯环的密度–显示了含苯基的硅烷。氢键SiH⋅⋅⋅HO和OH⋅⋅⋅π(PH)是相似的强度,但可以在ν来区分的SiH范围:在ν SiH⋅⋅⋅HO振动出现在较低频率,而OH⋅⋅⋅π(Ph)配合物使Si–H振动移至较高频率。计算结果显示了苯硅烷氢键键合配合物中非共价相互作用的多面性。由于OH⋅⋅⋅HSi键较弱,其他非共价相互作用在分子间复合物的稳定化中竞争。尽管如此,苯基硅烷的“纯” DHB配合物的结构和电子参数与Et 3 SiH相似。



























 京公网安备 11010802027423号
京公网安备 11010802027423号