Journal of Cleaner Production ( IF 11.1 ) Pub Date : 2018-08-16 , DOI: 10.1016/j.jclepro.2018.08.118 Mayra A. Nascimento , Jean C. Cruz , Guilherme D. Rodrigues , André F. de Oliveira , Renata P. Lopes
|
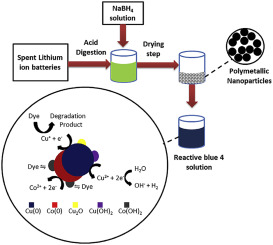
In this study inedited polymetallic nanoparticles were synthesized from electronic wastes of spent lithium-ion batteries (PN-LIB) and applied in the removal of the dye reactive blue 4 (RB4). Initially, the extraction of metals from batteries was performed using HCl/HNO3 (3:1 v/v). Subsequently, the PN-LIB were synthesized via chemical reduction with NaBH4. The PN-LIB showed spherical shape and size smaller than 50 nm, being constituted by Cu, Co, Ni, and Mn. The surface area was estimated at 92.29 m2 g−1 and the volume and average size of pores in 0.25 cm3 g−1 and 5.84 nm, respectively. The X-Rays diffractogram presented characteristic peaks of the Cu0. The PN-LIB were applied in the removal of the RB4 dye, in which the effects caused by the variables initial pH, dose of PN-LIB and initial concentration of dye in the removal process were evaluated. There was no significant effect of initial pH on the RB4 removal, since, a natural increase of this parameter occurred in the system and maintained at pH about 8.5. The rate of removal increases with the increase in the dose of PN-LIB and with the reduction of the initial concentration of dye. The experimental data were adjusted to the kinetic model of pseudo-second order and the Langmuir isotherm model, with a maximum capacity of removal, was calculated as being equal to 344.83 mg g−1. The molecular absorption spectra in the UV/Vis region and the infrared spectra indicated that two phenomena happen, adsorption and degradation of the dye.
中文翻译:

由废锂离子电池合成多金属纳米颗粒及其在去除活性蓝4染料中的应用
在这项研究中,用废锂离子电池(PN-LIB)的电子废料合成了未编辑的多金属纳米粒子,并将其应用于染料活性蓝4(RB4)的去除。最初,使用HCl / HNO 3(3:1 v / v)从电池中提取金属。随后,通过用NaBH 4进行化学还原来合成PN-LIB 。PN-LIB显示球形并且尺寸小于50nm,由Cu,Co,Ni和Mn构成。表面积估计为92.29 m 2 g -1,孔的体积和平均尺寸分别为0.25 cm 3 g -1和5.84 nm。X射线衍射图显示了Cu 0的特征峰。将PN-LIB应用于RB4染料的去除,其中评估了变量pH值,PN-LIB剂量和染料在去除过程中的初始浓度等变量引起的影响。初始pH值对RB4的去除没有显着影响,因为该参数在系统中自然增加并保持在约8.5的pH值。去除速率随着PN-LIB剂量的增加以及染料初始浓度的降低而增加。将实验数据调整为拟二阶动力学模型,计算出最大去除容量的Langmuir等温模型等于344.83 mg g -1。UV / Vis区域的分子吸收光谱和红外光谱表明发生了两种现象,即染料的吸附和降解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号