当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Gelation of rapeseed protein and whey protein mixtures at neutral pH and two heating temperatures: The impact of mixing on rheological and water holding properties
Food Hydrocolloids ( IF 10.7 ) Pub Date : 2019-02-01 , DOI: 10.1016/j.foodhyd.2018.08.023 William Nicholas Ainis , Carsten Ersch , Camille Farinet , Qiuhuizi Yang , Zachary J. Glover , Richard Ipsen
Food Hydrocolloids ( IF 10.7 ) Pub Date : 2019-02-01 , DOI: 10.1016/j.foodhyd.2018.08.023 William Nicholas Ainis , Carsten Ersch , Camille Farinet , Qiuhuizi Yang , Zachary J. Glover , Richard Ipsen
|
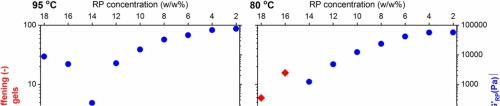
Abstract Whey proteins (WP) were replaced with rapeseed proteins (RP) at various protein mixing ratios at neutral pH and ionic strength of 150 mM. Protein gels were formed at 95 °C and another temperature of 80 °C, below the denaturation temperature of RP, to understand the importance of whether RP gelled or not for changes in rheological properties in mixed gels. Gels were analyzed for their microstructure, rheological responses (e.g. G') and water holding (maximum amount of exuded water (Amax) and ease with which water can be exuded (k)). The rheological responses obtained for mixed gels was compared to the ones of the single systems at the corresponding concentrations to understand the importance of the rheological moduli of the single systems to the modulus of the mixed gel and to enable the quantification of synergistic stiffening (S = G΄mixture/(G΄WP + G΄RP)). For gels formed at 95 °C a broader window for synergistic stiffening was formed compared to gels formed at 80 °C. Synergistic stiffening occurred when gel coarseness decreased and/or when the differences between the moduli of the individual protein gels were minimized. Water holding properties of mixed gels were tailored due to changes in the gel microstructure or due to synergistic stiffening. The behavior of k as a function of protein mixing ratio in mixed gels was similar to the behavior of gel stiffness. The decrease of k correlated well with an increase in gel stiffness. Gel coarseness and gel stiffness were inextricably linked in regards to their importance for changes in Amax.
中文翻译:

油菜籽蛋白和乳清蛋白混合物在中性 pH 值和两个加热温度下的凝胶化:混合对流变和保水性能的影响
摘要 在中性 pH 值和 150 mM 离子强度下,乳清蛋白 (WP) 被油菜籽蛋白 (RP) 以不同的蛋白质混合比例取代。蛋白质凝胶在 95 °C 和 80 °C 的另一个温度下形成,低于 RP 的变性温度,以了解 RP 是否胶凝对混合凝胶中流变特性变化的重要性。分析了凝胶的微观结构、流变学响应(例如 G')和保水性(最大渗出水量 (Amax) 和渗出水的难易程度 (k))。将混合凝胶获得的流变响应与相应浓度的单一系统的流变响应进行比较,以了解单一系统的流变模量对混合凝胶模量的重要性,并能够量化协同硬化 (S = G΄混合物/(G΄WP + G΄RP))。对于在 95 °C 形成的凝胶,与在 80 °C 形成的凝胶相比,形成了更宽的协同硬化窗口。当凝胶粗糙度降低和/或当单个蛋白质凝胶的模量之间的差异最小化时,就会发生协同硬化。由于凝胶微观结构的变化或由于协同硬化,混合凝胶的保水性能被调整。作为混合凝胶中蛋白质混合比的函数的 k 的行为类似于凝胶刚度的行为。k 的降低与凝胶刚度的增加密切相关。凝胶粗糙度和凝胶硬度与它们对 Amax 变化的重要性有着密不可分的联系。
更新日期:2019-02-01
中文翻译:

油菜籽蛋白和乳清蛋白混合物在中性 pH 值和两个加热温度下的凝胶化:混合对流变和保水性能的影响
摘要 在中性 pH 值和 150 mM 离子强度下,乳清蛋白 (WP) 被油菜籽蛋白 (RP) 以不同的蛋白质混合比例取代。蛋白质凝胶在 95 °C 和 80 °C 的另一个温度下形成,低于 RP 的变性温度,以了解 RP 是否胶凝对混合凝胶中流变特性变化的重要性。分析了凝胶的微观结构、流变学响应(例如 G')和保水性(最大渗出水量 (Amax) 和渗出水的难易程度 (k))。将混合凝胶获得的流变响应与相应浓度的单一系统的流变响应进行比较,以了解单一系统的流变模量对混合凝胶模量的重要性,并能够量化协同硬化 (S = G΄混合物/(G΄WP + G΄RP))。对于在 95 °C 形成的凝胶,与在 80 °C 形成的凝胶相比,形成了更宽的协同硬化窗口。当凝胶粗糙度降低和/或当单个蛋白质凝胶的模量之间的差异最小化时,就会发生协同硬化。由于凝胶微观结构的变化或由于协同硬化,混合凝胶的保水性能被调整。作为混合凝胶中蛋白质混合比的函数的 k 的行为类似于凝胶刚度的行为。k 的降低与凝胶刚度的增加密切相关。凝胶粗糙度和凝胶硬度与它们对 Amax 变化的重要性有着密不可分的联系。


























 京公网安备 11010802027423号
京公网安备 11010802027423号