Acta Biomaterialia ( IF 9.7 ) Pub Date : 2018-08-15 , DOI: 10.1016/j.actbio.2018.08.012 Pieterjan Merckx , Lynn De Backer , Lien Van Hoecke , Roberta Guagliardo , Mercedes Echaide , Pieter Baatsen , Bárbara Olmeda , Xavier Saelens , Jésus Pérez-Gil , Stefaan C. De Smedt , Koen Raemdonck
|
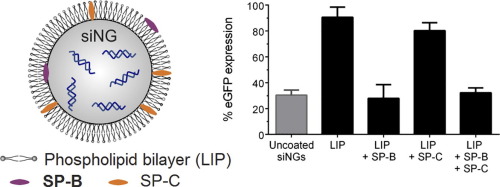
Despite the many advantages of small interfering RNA (siRNA) inhalation therapy and a growing prevalence for respiratory pathologies, its clinical translation is severely hampered by inefficient intracellular delivery. To this end, we previously developed hybrid nanoparticles consisting of an siRNA-loaded nanosized hydrogel core (nanogel) coated with Curosurf®, a clinically used pulmonary surfactant (PS). Interestingly, the PS shell was shown to markedly improve particle stability as well as intracellular siRNA delivery in vitro and in vivo. The major aim of this work was to identify the key molecular components of PS responsible for the enhanced siRNA delivery and evaluate how the complexity of the PS coat could be reduced. We identified surfactant protein B (SP-B) as a potent siRNA delivery enhancer when reconstituted in proteolipid coated hydrogel nanocomposites. Improved cytosolic siRNA delivery was achieved by inserting SP-B into a simplified phospholipid mixture prior to nanogel coating. This effect was observed both in vitro (lung epithelial cell line) and in vivo (murine acute lung injury model), albeit that distinct phospholipids were required to achieve these results. Importantly, the developed nanocomposites have a low in vivo toxicity and are efficiently taken up by resident alveolar macrophages, a main target cell type for treatment of inflammatory pulmonary pathologies. Our results demonstrate the potential of the endogenous protein SP-B as an intracellular siRNA delivery enhancer, paving the way for future design of nanoformulations for siRNA inhalation therapy.
Statement of significance
Despite the therapeutic potential of small interfering RNA (siRNA) and a growing prevalence of lung diseases for which innovative therapies are needed, a safe and effective siRNA inhalation therapy remains non-existing due to a lack of suitable formulations. We identified surfactant protein B (SP-B) as a potent enhancer of siRNA delivery by proteolipid coated nanogel formulations in vitro in a lung epithelial cell line. The developed nanocomposites have a low in vivo toxicity and show a high uptake by alveolar macrophages, a main target cell type for treatment of inflammatory pulmonary pathologies. Importantly, in vivo SP-B is also critical for the developed formulation to obtain a significant silencing of TNF α in a murine LPS-induced acute lung injury model.
中文翻译:

表面活性剂蛋白b(sp-b)增强了用于吸入疗法的蛋白脂质包被的纳米凝胶的细胞膜irna传递
尽管小干扰RNA(siRNA)吸入疗法具有许多优势,并且呼吸道疾病的患病率不断上升,但其细胞内传递效率低下严重阻碍了其临床翻译。为此,我们以前开发的由涂覆有固尔苏的siRNA加载纳米尺寸的水凝胶芯(纳米凝胶)的混合纳米粒子®,一个临床使用的肺表面活性物质(PS)。有趣的是,PS壳层可显着改善体外和体内颗粒的稳定性以及细胞内siRNA的递送。这项工作的主要目的是确定负责增强siRNA传递的PS的关键分子成分,并评估如何降低PS涂层的复杂性。我们将表面活性剂蛋白B(SP-B)确定为有效的siRNA传递增强剂,当在蛋白脂质体包覆的水凝胶纳米复合材料中重构时。通过在纳米凝胶涂层之前将SP-B插入简化的磷脂混合物中,可以改善胞质siRNA的递送。在体外(肺上皮细胞系)和体内(鼠急性肺损伤模型)均观察到了这种效果,尽管需要不同的磷脂才能达到这些结果。重要的是,已开发的纳米复合材料的体内活性低毒性,并被驻留的肺泡巨噬细胞有效吸收,肺泡巨噬细胞是治疗炎症性肺病的主要靶细胞类型。我们的结果证明了内源性蛋白SP-B作为细胞内siRNA传递增强剂的潜力,为将来设计用于siRNA吸入疗法的纳米制剂铺平了道路。
重要声明
尽管小干扰RNA(siRNA)具有治疗潜力,并且需要创新疗法的肺部疾病患病率正在上升,但是由于缺乏合适的制剂,安全,有效的siRNA吸入疗法仍然不存在。我们确定表面活性剂蛋白B(SP-B)是由蛋白脂质体包覆的纳米凝胶制剂在肺上皮细胞系中体外表达的有效siRNA增强剂。发达的纳米复合材料具有较低的体内毒性,并显示出肺泡巨噬细胞对肺炎巨噬细胞的摄取,肺泡巨噬细胞是治疗炎症性肺病的主要靶细胞类型。重要的是,体内 SP-B对于在小鼠LPS诱导的急性肺损伤模型中获得显着的TNFα沉默,对于开发的制剂也至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号