当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Versatile Bioconjugation Chemistries of ortho-Boronyl Aryl Ketones and Aldehydes
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-08-15 00:00:00 , DOI: 10.1021/acs.accounts.8b00154 Samantha Cambray 1 , Jianmin Gao 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-08-15 00:00:00 , DOI: 10.1021/acs.accounts.8b00154 Samantha Cambray 1 , Jianmin Gao 1
Affiliation
|
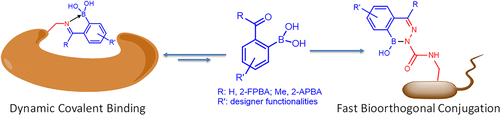
Biocompatible and bioorthogonal conjugation reactions have proven to be powerful tools in biological research and medicine. While the advent of bioorthogonal conjugation chemistries greatly expands our capacity to interrogate specific biomolecules in situ, biocompatible reactions that target endogenous reactive groups have given rise to a number of covalent drugs as well as a battery of powerful research tools. Despite the impressive progress, limitations do exist with the current conjugation chemistries. For example, most known bioorthogonal conjugations suffer from slow reaction rates and imperfect bioorthogonality. On the other hand, covalent drugs often display high toxicity due to off-target labeling and immunogenicity. These limitations demand continued pursuit of conjugation chemistries with optimal characteristics for biological applications. A spate of papers appearing in recent literature report the conjugation chemistries of 2-formyl and 2-acetyl phenylboronic acids (abbreviated as 2-FPBA and 2-APBA, respectively). These simple reactants are found to undergo fast conjugation with various nucleophiles under physiological conditions, showing great promise for biological applications.
中文翻译:

邻硼烷基芳基酮和醛的多功能生物共轭化学
生物相容性和生物正交共轭反应已被证明是生物学研究和医学中的有力工具。尽管生物正交共轭化学的出现极大地扩展了我们就地探询特定生物分子的能力,但靶向内源性反应基团的生物相容性反应却催生了许多共价药物以及一系列强大的研究工具。尽管取得了令人瞩目的进步,但是目前的缀合化学仍然存在局限性。例如,大多数已知的生物正交共轭具有慢的反应速率和不完美的生物正交性。另一方面,由于脱靶标记和免疫原性,共价药物通常表现出高毒性。这些限制要求继续追求具有用于生物学应用的最佳特性的结合化学。在最近的文献中出现的大量论文报道了2-甲酰基和2-乙酰基苯基硼酸(分别简称为2-FPBA和2-APBA)的结合化学。发现这些简单的反应物在生理条件下与各种亲核试剂快速结合,对生物学应用显示出很大的希望。
更新日期:2018-08-15
中文翻译:

邻硼烷基芳基酮和醛的多功能生物共轭化学
生物相容性和生物正交共轭反应已被证明是生物学研究和医学中的有力工具。尽管生物正交共轭化学的出现极大地扩展了我们就地探询特定生物分子的能力,但靶向内源性反应基团的生物相容性反应却催生了许多共价药物以及一系列强大的研究工具。尽管取得了令人瞩目的进步,但是目前的缀合化学仍然存在局限性。例如,大多数已知的生物正交共轭具有慢的反应速率和不完美的生物正交性。另一方面,由于脱靶标记和免疫原性,共价药物通常表现出高毒性。这些限制要求继续追求具有用于生物学应用的最佳特性的结合化学。在最近的文献中出现的大量论文报道了2-甲酰基和2-乙酰基苯基硼酸(分别简称为2-FPBA和2-APBA)的结合化学。发现这些简单的反应物在生理条件下与各种亲核试剂快速结合,对生物学应用显示出很大的希望。

























 京公网安备 11010802027423号
京公网安备 11010802027423号