Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-08-15 , DOI: 10.1016/j.bioorg.2018.08.020 Didem Şöhretoğlu , Suat Sari , Burak Barut , Arzu Özel
|
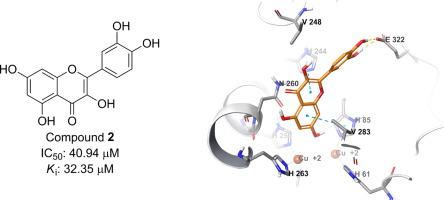
Flavonoids are main polyphenolic groups widely distributed to fruits, vegetables and beverages we consumed daily. They exhibit many biological effects. We tested tyrosinase inhibitor potential of structurally related (1–9) flavonoids and found that all the tested materials possessed tyrosinase inhibitory effect compared to the positive control, kojic acid. 2 exhibited the strongest tyrosinase inhibitory effect with an IC50 value of 40.94 ± 0.78 µM in a competitive manner. According to kinetic analysis 1, 4 and 7 were found to be competitive inhibitors, 3, 5, and 6 noncompetitive inhibitors of tyrosinase. According to the docking studies, A and C ring of the flavonoid structure, hydroxyl substituent at the 7th position, and hydroxyl substituents at para or para and meta position of ring B play key role for competitive inhibition of the enzyme.
中文翻译:

某些类黄酮对酪氨酸酶的抑制作用:体外和计算机研究的抑制活性,机理
黄酮类化合物是主要的多酚基团,广泛分布于我们日常消费的水果,蔬菜和饮料中。它们表现出许多生物学效应。我们测试了结构上相关(的酪氨酸酶抑制剂潜在1 - 9)黄酮类化合物,发现所有测试的材料所具有酪氨酸酶抑制效果相比,阳性对照,曲酸。2以竞争性方式表现出最强的酪氨酸酶抑制作用,IC 50值为40.94±0.78 µM。根据动力学分析1,4和7被认为是竞争性抑制剂,3,5,和6酪氨酸酶的非竞争性抑制剂。根据对接研究,类黄酮结构的A和C环,在B环的第7位的羟基取代基,以及在环B的对位或对位和间位的羟基取代基在竞争抑制该酶中起关键作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号